Saliva Alternative to Upper Respiratory Swabs for SARS-CoV-2 Diagnosis
- PMID: 32917294
- PMCID: PMC7588522
- DOI: 10.3201/eid2611.203283
Saliva Alternative to Upper Respiratory Swabs for SARS-CoV-2 Diagnosis
Abstract
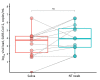
PCR of upper respiratory specimens is the diagnostic standard for severe acute respiratory syndrome coronavirus 2 infection. However, saliva sampling is an easy alternative to nasal and throat swabbing. We found similar viral loads in saliva samples and in nasal and throat swab samples from 110 patients with coronavirus disease.
Keywords: 2019 novel coronavirus disease; COVID-19; RT-qPCR; SARS-CoV-2; coronavirus; coronavirus disease; diagnosis; respiratory infections; saliva; severe acute respiratory syndrome coronavirus 2; swabs; testing; upper respiratory swab samples; viruses; zoonoses.
Figures
Similar articles
-
Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study.Lancet Infect Dis. 2020 May;20(5):565-574. doi: 10.1016/S1473-3099(20)30196-1. Epub 2020 Mar 23. Lancet Infect Dis. 2020. PMID: 32213337 Free PMC article.
-
Self-Collected Anterior Nasal and Saliva Specimens versus Health Care Worker-Collected Nasopharyngeal Swabs for the Molecular Detection of SARS-CoV-2.J Clin Microbiol. 2020 Oct 21;58(11):e01824-20. doi: 10.1128/JCM.01824-20. Print 2020 Oct 21. J Clin Microbiol. 2020. PMID: 32817233 Free PMC article.
-
Sample collection and stabilization from saliva samples for SARS-CoV-2 detection by qPCR.Biotechniques. 2020 Sep;69(3):237. doi: 10.2144/btn-2020-1001ap. Biotechniques. 2020. PMID: 32929994 No abstract available.
-
New COVID-19 saliva-based test: How good is it compared with the current nasopharyngeal or throat swab test?J Chin Med Assoc. 2020 Oct;83(10):891-894. doi: 10.1097/JCMA.0000000000000396. J Chin Med Assoc. 2020. PMID: 32773584 Free PMC article. Review.
-
[SARS-CoV-2 and Microbiological Diagnostic Dynamics in COVID-19 Pandemic].Mikrobiyol Bul. 2020 Jul;54(3):497-509. doi: 10.5578/mb.69839. Mikrobiyol Bul. 2020. PMID: 32755524 Review. Turkish.
Cited by
-
SalivaDirect: an alternative to a conventional RNA extraction protocol for molecular detection of SARS-CoV-2 in a clinical setting.Microbiol Spectr. 2024 Jan 11;12(1):e0327223. doi: 10.1128/spectrum.03272-23. Epub 2023 Nov 28. Microbiol Spectr. 2024. PMID: 38014980 Free PMC article.
-
Influenza A, like Omicron SARS-CoV-2, Is Similarly Detected in Saliva or Nasopharyngeal Samples via RT-qPCR.Viruses. 2023 Nov 30;15(12):2352. doi: 10.3390/v15122352. Viruses. 2023. PMID: 38140593 Free PMC article.
-
Population wide testing pooling strategy for SARS-CoV-2 detection using saliva.PLoS One. 2022 Jan 28;17(1):e0263033. doi: 10.1371/journal.pone.0263033. eCollection 2022. PLoS One. 2022. PMID: 35089942 Free PMC article.
-
Robotic RNA extraction for SARS-CoV-2 surveillance using saliva samples.PLoS One. 2021 Aug 5;16(8):e0255690. doi: 10.1371/journal.pone.0255690. eCollection 2021. PLoS One. 2021. PMID: 34351984 Free PMC article.
-
Optimization and Improvement of qPCR Detection Sensitivity of SARS-CoV-2 in Saliva.Microbiol Spectr. 2023 Jun 15;11(3):e0464022. doi: 10.1128/spectrum.04640-22. Epub 2023 Apr 25. Microbiol Spectr. 2023. PMID: 37097200 Free PMC article.
References
-
- World Health Organization. Laboratory testing strategy recommendations for COVID-19. 2020 [cited 2020 July 1]. https://www.who.int/publications/i/item/laboratory-testing-strategy-reco...
-
- Centers for Disease Control and Prevention. Interim guidelines for collecting, handling, and testing clinical specimens from persons for coronavirus disease 2019 (COVID-19). 2020. [cited 2020 Jun 22]. https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specim...
-
- To KKW, Tsang OTY, Leung WS, Tam AR, Wu TC, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–74. 10.1016/S1473-3099(20)30196-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

