Novel fractionated ultrashort thermal exposures with MRI-guided focused ultrasound for treating tumors with thermosensitive drugs
- PMID: 32917589
- PMCID: PMC7467687
- DOI: 10.1126/sciadv.aba5684
Novel fractionated ultrashort thermal exposures with MRI-guided focused ultrasound for treating tumors with thermosensitive drugs
Abstract
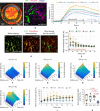
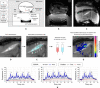
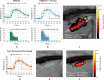
Thermosensitive liposomes represent an important paradigm in oncology, where hyperthermia-mediated release coupled with thermal bioeffects enhance the effectiveness of chemotherapy. Their widespread clinical adoption hinges upon performing controlled targeted hyperthermia, and a leading candidate to achieve this is temperature-based magnetic resonance imaging (MRI)-guided focused ultrasound (MRgFUS). However, the current approach to hyperthermia involves exposures lasting tens of minutes to hours, which is not possible to achieve in many circumstances because of blood vessel cooling and respiratory motion. Here, we investigate a novel approach to overcome these limitations: to use fractionated ultrashort (~30 s) thermal exposures (~41° to 45°C) to release doxorubicin from a thermosensitive liposome. This is first demonstrated in a dorsal chamber tumor model using two-photon microscopy. Thermal exposures were then conducted with a rabbit tumor model using a custom MRgFUS system incorporating temperature feedback control. Drug release was confirmed, and longitudinal experiments demonstrated profoundly enhanced tumor growth inhibition and survival.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- Yatvin M. B., Weinstein J. N., Dennis W. H., Blumenthal R., Design of liposomes for enhanced local release of drugs by hyperthermia. Science 202, 1290–1293 (1978). - PubMed
-
- Stapleton S., Dunne M., Milosevic M., Tran C. W., Gold M. J., Vedadi A., Mckee T. D., Ohashi P. S., Allen C., Jaffray D. A., Radiation and heat improve the delivery and efficacy of nanotherapeutics by modulating intratumoral fluid dynamics. ACS Nano 12, 7583–7600 (2018). - PubMed
-
- Kong G., Braun R. D., Dewhirst M. W., Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Res. 60, 4440–4445 (2000). - PubMed
-
- Sen A., Capitano M. L., Spernyak J. A., Schueckler J. T., Thomas S., Singh A. K., Evans S. S., Hylander B. L., Repasky E. A., Mild elevation of body temperature reduces tumor interstitial fluid pressure and hypoxia and enhances efficacy of radiotherapy in murine tumor models. Cancer Res. 71, 3872–3880 (2011). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

