Dose-dependent effects of transcranial alternating current stimulation on spike timing in awake nonhuman primates
- PMID: 32917605
- PMCID: PMC7467690
- DOI: 10.1126/sciadv.aaz2747
Dose-dependent effects of transcranial alternating current stimulation on spike timing in awake nonhuman primates
Abstract
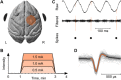
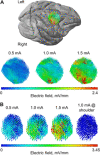
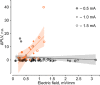
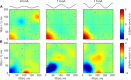
Weak extracellular electric fields can influence spike timing in neural networks. Approaches to noninvasively impose these fields on the brain have high therapeutic potential in neurology and psychiatry. Transcranial alternating current stimulation (TACS) is hypothesized to affect spike timing and cause neural entrainment. However, the conditions under which these effects occur in vivo are unknown. Here, we recorded single-unit activity in the neocortex in awake nonhuman primates during TACS and found dose-dependent neural entrainment to the stimulation waveform. Cluster analysis of changes in interspike intervals identified two main types of neural responses to TACS-increased burstiness and phase entrainment. Our results uncover key mechanisms of TACS and show that the stimulation affects spike timing in the awake primate brain at intensities feasible in humans. Thus, novel TACS protocols tailored to ongoing brain activity may be a tool to normalize spike timing in maladaptive brain networks and neurological disease.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



Comment in
-
Can low-intensity tACS genuinely entrain neural activity in vivo?Brain Stimul. 2020 Nov-Dec;13(6):1796-1799. doi: 10.1016/j.brs.2020.10.002. Epub 2020 Oct 9. Brain Stimul. 2020. PMID: 33045404
References
-
- Singer W., Neuronal oscillations: Unavoidable and useful? Eur. J. Neurosci. 48, 2389–2398 (2018). - PubMed
-
- Anastassiou C. A., Perin R., Markram H., Koch C., Ephaptic coupling of cortical neurons. Nat. Neurosci. 14, 217–223 (2011). - PubMed
-
- Anastassiou C. A., Koch C., Ephaptic coupling to endogenous electric field activity: Why bother? Curr. Opin. Neurobiol. 31, 95–103 (2015). - PubMed
-
- Borst A., Theunissen F. E., Information theory and neural coding. Nat. Neurosci. 2, 947–957 (1999). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

