3D bioprinting dual-factor releasing and gradient-structured constructs ready to implant for anisotropic cartilage regeneration
- PMID: 32917692
- PMCID: PMC11206535
- DOI: 10.1126/sciadv.aay1422
3D bioprinting dual-factor releasing and gradient-structured constructs ready to implant for anisotropic cartilage regeneration
Abstract
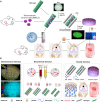
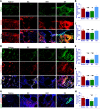
Cartilage injury is extremely common and leads to joint dysfunction. Existing joint prostheses do not remodel with host joint tissue. However, developing large-scale biomimetic anisotropic constructs mimicking native cartilage with structural integrity is challenging. In the present study, we describe anisotropic cartilage regeneration by three-dimensional (3D) bioprinting dual-factor releasing and gradient-structured constructs. Dual-factor releasing mesenchymal stem cell (MSC)-laden hydrogels were used for anisotropic chondrogenic differentiation. Together with physically gradient synthetic biodegradable polymers that impart mechanical strength, the 3D bioprinted anisotropic cartilage constructs demonstrated whole-layer integrity, lubrication of superficial layers, and nutrient supply in deep layers. Evaluation of the cartilage tissue in vitro and in vivo showed tissue maturation and organization that may be sufficient for translation to patients. In conclusion, one-step 3D bioprinted dual-factor releasing and gradient-structured constructs were generated for anisotropic cartilage regeneration, integrating the feasibility of MSC- and 3D bioprinting-based therapy for injured or degenerative joints.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution License 4.0 (CC BY).
Figures




References
-
- Merriam A. R., Patel J. M., Culp B. M., Gatt C. J. Jr., Dunn M. G., Successful Total Meniscus Reconstruction Using a Novel Fiber-Reinforced Scaffold: A 16- and 32-Week Study in an Ovine Model. Am. J. Sports Med. 43, 2528–2537 (2015). - PubMed
-
- Ayers D. C., How Common Is Revision for Adverse Reaction to Metal Debris After Total Hip Replacement with a Metal-on-Polyethylene Bearing Surface?: Commentary on an article by Anders Persson, MD, et al.: "Revision for Symptomatic Pseudotumor Following Primary Total Hip Arthroplasty with a Standard Femoral Stem". J. Bone Joint Surg. Am. 100, e82 (2018). - PubMed
-
- Craft A. M., Rockel J. S., Nartiss Y., Kandel R. A., Alman B. A., Keller G. M., Generation of articular chondrocytes from human pluripotent stem cells. Nat. Biotechnol. 33, 638–645 (2015). - PubMed
Publication types
LinkOut - more resources
Full Text Sources

