Real-time nanodiamond thermometry probing in vivo thermogenic responses
- PMID: 32917703
- PMCID: PMC7486095
- DOI: 10.1126/sciadv.aba9636
Real-time nanodiamond thermometry probing in vivo thermogenic responses
Abstract
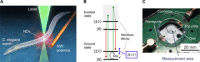
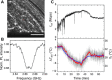
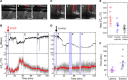
Real-time temperature monitoring inside living organisms provides a direct measure of their biological activities. However, it is challenging to reduce the size of biocompatible thermometers down to submicrometers, despite their potential applications for the thermal imaging of subtissue structures with single-cell resolution. Here, using quantum nanothermometers based on optically accessible electron spins in nanodiamonds, we demonstrate in vivo real-time temperature monitoring inside Caenorhabditis elegans worms. We developed a microscope system that integrates a quick-docking sample chamber, particle tracking, and an error correction filter for temperature monitoring of mobile nanodiamonds inside live adult worms with a precision of ±0.22°C. With this system, we determined temperature increases based on the worms' thermogenic responses during the chemical stimuli of mitochondrial uncouplers. Our technique demonstrates the submicrometer localization of temperature information in living animals and direct identification of their pharmacological thermogenesis, which may allow for quantification of their biological activities based on temperature.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution License 4.0 (CC BY).
Figures

References
-
- Akin J. A., Homeostatic processes for thermoregulation. Nat. Educ. Knowledge 3, 7 (2011).
-
- Marusyk A., Almendro V., Polyak K., Intra-tumour heterogeneity: A looking glass for cancer? Nat. Rev. Cancer 12, 323–334 (2012). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

