Dimorphism in cryptophytes-The case of Teleaulax amphioxeia/ Plagioselmis prolonga and its ecological implications
- PMID: 32917704
- PMCID: PMC7486100
- DOI: 10.1126/sciadv.abb1611
Dimorphism in cryptophytes-The case of Teleaulax amphioxeia/ Plagioselmis prolonga and its ecological implications
Abstract
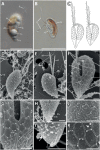
Growing evidence suggests that sexual reproduction might be common in unicellular organisms, but observations are sparse. Limited knowledge of sexual reproduction constrains understanding of protist ecology. Although Teleaulax amphioxeia and Plagioselmis prolonga are common marine cryptophytes worldwide, and are also important plastid donors for some kleptoplastic ciliates and dinoflagellates, the ecology and development of these protists are poorly known. We demonstrate that P. prolonga is the haploid form of the diploid T. amphioxeia and describe the seasonal dynamics of these two life stages. The diploid T. amphioxeia dominates during periods of high dissolved inorganic nitrogen (DIN) and low irradiance, temperature, and grazing (winter and early spring), whereas the haploid P. prolonga becomes more abundant during the summer, when DIN is low and irradiance, temperature, and grazing are high. Dimorphic sexual life cycles might explain the success of this species by fostering high genetic diversity and enabling endurance in adverse conditions.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures




References
-
- Cerino F., Zingone A., A survey of cryptomonad diversity and seasonality at a coastal Mediterranean site. Eur. J. Phycol. 41, 363–378 (2006).
-
- Gustafson D. E., Stoecker D. K., Johnson M. D., Van Heukelem W. F., Sneider K., Cryptophyte algae are robbed of their organelles by the marine ciliate Mesodinium rubrum. Nature 405, 1049–1052 (2000). - PubMed
-
- Hansen P. J., Moldrup M., Tarangkoon W., Garcia-Cuetos L., Moestrup Ø., Direct evidence for symbiont sequestration in the marine red tide ciliate Mesodinium rubrum. Aquat. Microb. Ecol. 66, 63–75 (2012).
-
- Koike K., Takishita K., Anucleated cryptophyte vestiges in the gonyaulacalean dinoflagellates Amylax buxus and Amylax triacantha (Dinophyceae). Phycol. Res. 56, 301–311 (2008).
-
- Park M. G., Kim M., Kang M., A dinoflagellate Amylax triacantha with plastids of the cryptophyte origin: phylogeny, feeding mechanism, and growth and grazing responses. J. Eukaryot. Microbiol. 60, 363–376 (2013). - PubMed
Publication types
LinkOut - more resources
Full Text Sources

