Stimulation of ABCB4/MDR3 ATPase activity requires an intact phosphatidylcholine lipid
- PMID: 32917728
- PMCID: PMC7707170
- DOI: 10.1194/jlr.RA120000889
Stimulation of ABCB4/MDR3 ATPase activity requires an intact phosphatidylcholine lipid
Abstract
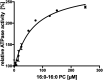
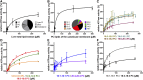
ABCB4/MDR3 is located in the canalicular membrane of hepatocytes and translocates PC-lipids from the cytoplasmic to the extracellular leaflet. ABCB4 is an ATP-dependent transporter that reduces the harsh detergent effect of the bile salts by counteracting self-digestion. To do so, ABCB4 provides PC lipids for extraction into bile. PC lipids account for 40% of the entire pool of lipids in the canalicular membrane with an unknown distribution over both leaflets. Extracted PC lipids end up in so-called mixed micelles. Mixed micelles are composed of phospholipids, bile salts, and cholesterol. Ninety to ninety-five percent of the phospholipids are members of the PC family, but only a subset of mainly 16.0-18:1 PC and 16:0-18:2 PC variants are present. To elucidate whether ABCB4 is the key discriminator in this enrichment of specific PC lipids, we used in vitro studies to identify crucial determinants in substrate selection. We demonstrate that PC-lipid moieties alone are insufficient for stimulating ABCB4 ATPase activity, and that at least two acyl chains and the backbone itself are required for a productive interaction. The nature of the fatty acids, like length or saturation has a quantitative impact on the ATPase activity. Our data demonstrate a two-step enrichment and protective function of ABCB4 to mitigate the harsh detergent effect of the bile salts, because ABCB4 can translocate more than just the PC-lipid variants found in bile.
Keywords: ATP binding cassette subfamily B member 4; adenosine 5′-triphosphatase activity; adenosine 5′-triphosphate binding cassette transporter; bile; fatty acids.
Copyright © 2020 Prescher et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
-
- Elferink R. P., Tytgat G. N., and Groen A. K.. 1997. Hepatic canalicular membrane 1: the role of mdr2 P-glycoprotein in hepatobiliary lipid transport. FASEB J. 11: 19–28. - PubMed
-
- Smit J. J., Schinkel A. H., Elferink R. P., Groen A. K., Wagenaar E., van Deemter L., Mol C. A., Ottenhoff R., van der Lugt N. M., Roon M. A., et al. . 1993. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver-disease. Cell. 75: 451–462. - PubMed
-
- Smith A. J., de Vree J. M. L., Ottenhoff R., Elferink R. P. J. O., Schinkel A. H., and Borst P.. 1998. Hepatocyte-specific expression of the human MDR3 P-glycoprotein gene restores the biliary phosphatidylcholine excretion absent in Mdr2 (-/-) mice. Hepatology. 28: 530–536. - PubMed
-
- Puglielli L., Amigo L., Arrese M., Nunez L., Rigotti A., Garrido J., Gonzalez S., Mingrone G., Greco A. V., Accatino L., et al. . 1994. Protective role of biliary cholesterol and phospholipid lamellae against bile acid-induced cell-damage. Gastroenterology. 107: 244–254. - PubMed
-
- Guyot C., and Stieger B.. 2011. Interaction of bile salts with rat canalicular membrane vesicles: evidence for bile salt resistant microdomains. J. Hepatol. 55: 1368–1376. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

