Type II Cytokines Fine-Tune Thymic T Cell Selection to Offset Murine Central Nervous System Autoimmunity
- PMID: 32917785
- PMCID: PMC7541690
- DOI: 10.4049/jimmunol.2000614
Type II Cytokines Fine-Tune Thymic T Cell Selection to Offset Murine Central Nervous System Autoimmunity
Abstract
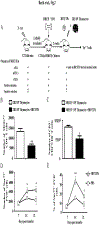
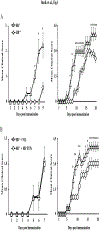
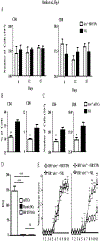
Early thymic progenitors (ETPs) are bone marrow-derived hematopoietic stem cells that remain multipotent and give rise to a variety of lineage-specific cells. Recently, we discovered a subset of murine ETPs that expresses the IL-4Rα/IL-13Rα1 heteroreceptor (HR) and commits only to the myeloid lineage. This is because IL-4/IL-13 signaling through the HR inhibits their T cell potential and enacts commitment of HR+ETPs to thymic resident CD11c+CD8α+ dendritic cells (DCs). In this study, we discovered that HR+-ETP-derived DCs function as APCs in the thymus and promote deletion of myelin-reactive T cells. Furthermore, this negative T cell selection function of HR+-ETP-derived DCs sustains protection against experimental allergic encephalomyelitis, a mouse model for human multiple sclerosis. These findings, while shedding light on the intricacies underlying ETP lineage commitment, reveal a novel, to our knowledge, function by which IL-4 and IL-13 cytokines condition thymic microenvironment to rheostat T cell selection and fine-tune central tolerance.
Copyright © 2020 by The American Association of Immunologists, Inc.
Figures

Similar articles
-
Reduced thymic IL-4 impairs negative T cell selection in nonobese diabetic mice.J Clin Invest. 2024 Dec 2;134(23):e163417. doi: 10.1172/JCI163417. J Clin Invest. 2024. PMID: 39621313 Free PMC article.
-
IL-4/IL-13 Signaling Inhibits the Potential of Early Thymic Progenitors To Commit to the T Cell Lineage.J Immunol. 2017 Oct 15;199(8):2767-2776. doi: 10.4049/jimmunol.1700498. Epub 2017 Sep 11. J Immunol. 2017. PMID: 28893952 Free PMC article.
-
IL-4 and IL-13 Guide Early Thymic Progenitors To Mature toward Dendritic Cells.J Immunol. 2018 Nov 15;201(10):2947-2958. doi: 10.4049/jimmunol.1701186. Epub 2018 Oct 5. J Immunol. 2018. PMID: 30291166 Free PMC article.
-
[T lymphocyte development and selection in the thymic microenvironments].Seikagaku. 2012 Mar;84(3):177-82. Seikagaku. 2012. PMID: 22568069 Review. Japanese. No abstract available.
-
A central role for peripheral dendritic cells in the induction of acquired thymic tolerance.Trends Immunol. 2003 Feb;24(2):77-81. doi: 10.1016/s1471-4906(02)00038-8. Trends Immunol. 2003. PMID: 12547504 Review.
Cited by
-
Reduced thymic IL-4 impairs negative T cell selection in nonobese diabetic mice.J Clin Invest. 2024 Dec 2;134(23):e163417. doi: 10.1172/JCI163417. J Clin Invest. 2024. PMID: 39621313 Free PMC article.
References
-
- Bell JJ, and Bhandoola A. 2008. The earliest thymic progenitors for T cells possess myeloid lineage potential. Nature 452: 764–767. - PubMed
-
- Wada H, Masuda K, Satoh R, Kakugawa K, Ikawa T, Katsura Y, and Kawamoto H. 2008. Adult T-cell progenitors retain myeloid potential. Nature 452: 768–772. - PubMed
-
- Luc S, Luis TC, Boukarabila H, Macaulay IC, Buza-Vidas N, Bouriez-Jones T, Lutteropp M, Woll PS, Loughran SJ, Mead AJ, Hultquist A, Brown J, Mizukami T, Matsuoka S, Ferry H, Anderson K, Duarte S, Atkinson D, Soneji S, Domanski A, Farley A, Sanjuan-Pla A, Carella C, Patient R, de Bruijn M, Enver T, Nerlov C, Blackburn C, Godin I, and Jacobsen SE. 2012. The earliest thymic T cell progenitors sustain B cell and myeloid lineage potential. Nat Immunol 13: 412–419. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

