Perinatal androgens organize sex differences in mast cells and attenuate anaphylaxis severity into adulthood
- PMID: 32917815
- PMCID: PMC7519313
- DOI: 10.1073/pnas.1915075117
Perinatal androgens organize sex differences in mast cells and attenuate anaphylaxis severity into adulthood
Abstract
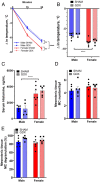
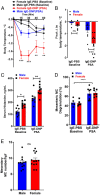
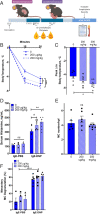
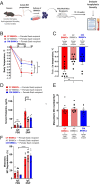
Mast cell (MC)-associated diseases, including allergy/anaphylaxis and neuroinflammatory pain disorders, exhibit a sex bias, with females at increase risk. While much attention has been directed toward adult sex hormones as drivers of sex differences, that female sex bias in MC-associated diseases is evident in prepubertal children, suggesting early-life origins of sex differences which have yet to be explored. Utilizing rodent models of MC-mediated anaphylaxis, our data here reveal that, 1) compared with females, males exhibit significantly reduced severity of MC-mediated anaphylactic responses that emerge prior to puberty and persist into adulthood, 2) reduced severity of MC-mediated anaphylaxis in males is linked with the naturally high level of perinatal androgens and can be recapitulated in females by perinatal exposure to testosterone proprionate, 3) perinatal androgen exposure guides bone marrow MC progenitors toward a masculinized tissue MC phenotype characterized by decreased concentration of prestored MC granule mediators (e.g., histamine, serotonin, and proteases) and reduced mediator release upon degranulation, and 4) engraftment of MC-deficient Kit W-sh/W-sh mice with adult male, female, or perinatally androgenized female MCs results in MC-mediated anaphylaxis response that reflects the MC sex and not host sex. Together, these data present evidence that sex differences in MC phenotype and resulting disease severity are established in early life by perinatal androgens. Thus, factors affecting levels of perinatal androgens could have a significant impact on MC development and MC-associated disease risk across the life span.
Keywords: histamine; immune; inflammation; mast cell; sex differences.
Conflict of interest statement
The authors declare no competing interest.
Figures



Similar articles
-
Mast cell corticotropin-releasing factor subtype 2 suppresses mast cell degranulation and limits the severity of anaphylaxis and stress-induced intestinal permeability.J Allergy Clin Immunol. 2019 May;143(5):1865-1877.e4. doi: 10.1016/j.jaci.2018.08.053. Epub 2018 Nov 12. J Allergy Clin Immunol. 2019. PMID: 30439403 Free PMC article.
-
Selective ablation of mast cells or basophils reduces peanut-induced anaphylaxis in mice.J Allergy Clin Immunol. 2013 Oct;132(4):881-8.e1-11. doi: 10.1016/j.jaci.2013.06.008. Epub 2013 Aug 1. J Allergy Clin Immunol. 2013. PMID: 23915716 Free PMC article.
-
Frontline Science: Corticotropin-releasing factor receptor subtype 1 is a critical modulator of mast cell degranulation and stress-induced pathophysiology.J Leukoc Biol. 2017 Dec;102(6):1299-1312. doi: 10.1189/jlb.2HI0317-088RR. Epub 2017 Jul 6. J Leukoc Biol. 2017. PMID: 28684600 Free PMC article.
-
Sex Differences in Mast Cell-Associated Disorders: A Life Span Perspective.Cold Spring Harb Perspect Biol. 2022 Oct 3;14(10):a039172. doi: 10.1101/cshperspect.a039172. Cold Spring Harb Perspect Biol. 2022. PMID: 35817512 Free PMC article. Review.
-
Mast Cell Disorders In Drug Hypersensitivity.Curr Pharm Des. 2016;22(45):6862-6869. doi: 10.2174/1381612822666160928121857. Curr Pharm Des. 2016. PMID: 27779084 Review.
Cited by
-
Overlapping conditions in Long COVID at a multisite academic center.Front Neurol. 2024 Oct 25;15:1482917. doi: 10.3389/fneur.2024.1482917. eCollection 2024. Front Neurol. 2024. PMID: 39524912 Free PMC article.
-
Early life adversity drives sex-specific anhedonia and meningeal immune gene expression through mast cell activation.Brain Behav Immun. 2022 Jul;103:73-84. doi: 10.1016/j.bbi.2022.03.009. Epub 2022 Mar 24. Brain Behav Immun. 2022. PMID: 35339629 Free PMC article.
-
Roles of IgE and Histamine in Mast Cell Maturation.Cells. 2021 Aug 23;10(8):2170. doi: 10.3390/cells10082170. Cells. 2021. PMID: 34440939 Free PMC article. Review.
-
Factors Influencing Marker Expressions of Cultured Human Cord Blood-Derived Mast Cells.Int J Mol Sci. 2023 Oct 4;24(19):14891. doi: 10.3390/ijms241914891. Int J Mol Sci. 2023. PMID: 37834338 Free PMC article.
-
Rumen and fecal microbiota profiles associated with immunity of young and adult goats.Front Immunol. 2022 Sep 13;13:978402. doi: 10.3389/fimmu.2022.978402. eCollection 2022. Front Immunol. 2022. PMID: 36177023 Free PMC article.
References
-
- Lovell R. M., Ford A. C., Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin. Gastroenterol. Hepatol. 10, 712–721.e4 (2012). - PubMed
-
- Gonzalez-Perez A., Aponte Z., Vidaurre C. F., Rodriguez L. A., Anaphylaxis epidemiology in patients with and patients without asthma: A United Kingdom database review. J. Allergy Clin. Immunol. 125, 1098–1104.e1 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

