In vivo neuroinflammation and cerebral small vessel disease in mild cognitive impairment and Alzheimer's disease
- PMID: 32917821
- PMCID: PMC7803899
- DOI: 10.1136/jnnp-2020-323894
In vivo neuroinflammation and cerebral small vessel disease in mild cognitive impairment and Alzheimer's disease
Abstract
Introduction: Associations between cerebral small vessel disease (SVD) and inflammation have been largely examined using peripheral blood markers of inflammation, with few studies measuring inflammation within the brain. We investigated the cross-sectional relationship between SVD and in vivo neuroinflammation using [11C]PK11195 positron emission tomography (PET) imaging.
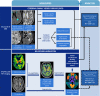
Methods: Forty-two participants were recruited (according to NIA-AA guidelines, 14 healthy controls, 14 mild Alzheimer's disease, 14 amyloid-positive mild cognitive impairment). Neuroinflammation was assessed using [11C]PK11195 PET imaging, a marker of microglial activation. To quantify SVD, we assessed white matter hyperintensities (WMH), enlarged perivascular spaces, cerebral microbleeds and lacunes. Composite scores were calculated for global SVD burden, and SVD subtypes of hypertensive arteriopathy and cerebral amyloid angiopathy (CAA). General linear models examined associations between SVD and [11C]PK11195, adjusting for sex, age, education, cognition, scan interval, and corrected for multiple comparisons via false discovery rate (FDR). Dominance analysis directly compared the relative importance of hypertensive arteriopathy and CAA scores as predictors of [11C]PK11195.
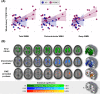
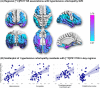
Results: Global [11C]PK11195 binding was associated with SVD markers, particularly in regions typical of hypertensive arteriopathy: deep microbleeds (β=0.63, F(1,35)=35.24, p<0.001), deep WMH (β=0.59, t=4.91, p<0.001). In dominance analysis, hypertensive arteriopathy score outperformed CAA in predicting [11C]PK11195 binding globally and in 28 out of 37 regions of interest, especially the medial temporal lobe (β=0.66-0.76, t=3.90-5.58, FDR-corrected p (pFDR)=<0.001-0.002) and orbitofrontal cortex (β=0.51-0.57, t=3.53-4.30, pFDR=0.001-0.004).
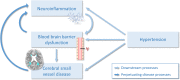
Conclusion: Microglial activation is associated with SVD, particularly with the hypertensive arteriopathy subtype of SVD. Although further research is needed to determine causality, our study suggests that targeting neuroinflammation might represent a novel therapeutic strategy for SVD.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
