Elucidating the role of metal ions in carbonic anhydrase catalysis
- PMID: 32917908
- PMCID: PMC7486293
- DOI: 10.1038/s41467-020-18425-5
Elucidating the role of metal ions in carbonic anhydrase catalysis
Abstract
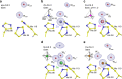
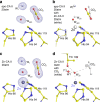
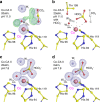
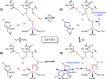
Why metalloenzymes often show dramatic changes in their catalytic activity when subjected to chemically similar but non-native metal substitutions is a long-standing puzzle. Here, we report on the catalytic roles of metal ions in a model metalloenzyme system, human carbonic anhydrase II (CA II). Through a comparative study on the intermediate states of the zinc-bound native CA II and non-native metal-substituted CA IIs, we demonstrate that the characteristic metal ion coordination geometries (tetrahedral for Zn2+, tetrahedral to octahedral conversion for Co2+, octahedral for Ni2+, and trigonal bipyramidal for Cu2+) directly modulate the catalytic efficacy. In addition, we reveal that the metal ions have a long-range (~10 Å) electrostatic effect on restructuring water network in the active site. Our study provides evidence that the metal ions in metalloenzymes have a crucial impact on the catalytic mechanism beyond their primary chemical properties.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Nucleophile recognition as an alternative inhibition mode for benzoic acid based carbonic anhydrase inhibitors.Chem Commun (Camb). 2012 May 28;48(43):5259-61. doi: 10.1039/c2cc32013d. Epub 2012 Apr 24. Chem Commun (Camb). 2012. PMID: 22531842 Free PMC article.
-
Metal binding specificity in carbonic anhydrase is influenced by conserved hydrophobic core residues.Biochemistry. 1999 Jul 13;38(28):9054-62. doi: 10.1021/bi9900166. Biochemistry. 1999. PMID: 10413479
-
Structural influence of hydrophobic core residues on metal binding and specificity in carbonic anhydrase II.Biochemistry. 2000 Nov 14;39(45):13687-94. doi: 10.1021/bi001649j. Biochemistry. 2000. PMID: 11076507
-
Interplay between Carbonic Anhydrases and Metallothioneins: Structural Control of Metalation.Int J Mol Sci. 2020 Aug 9;21(16):5697. doi: 10.3390/ijms21165697. Int J Mol Sci. 2020. PMID: 32784815 Free PMC article. Review.
-
Competition among metal ions for protein binding sites: determinants of metal ion selectivity in proteins.Chem Rev. 2014 Jan 8;114(1):538-56. doi: 10.1021/cr4004665. Epub 2013 Sep 16. Chem Rev. 2014. PMID: 24040963 Review. No abstract available.
Cited by
-
Characterization of an α-Amylase from the Honeybee Chalk Brood Pathogen Ascosphaera apis.J Fungi (Basel). 2023 Nov 5;9(11):1082. doi: 10.3390/jof9111082. J Fungi (Basel). 2023. PMID: 37998887 Free PMC article.
-
Fast product release requires active-site water dynamics in carbonic anhydrase.Nat Commun. 2025 May 12;16(1):4404. doi: 10.1038/s41467-025-59645-x. Nat Commun. 2025. PMID: 40355440 Free PMC article.
-
Genome-wide association and Mendelian randomization study of blood copper levels and 213 deep phenotypes in humans.Commun Biol. 2022 May 2;5(1):405. doi: 10.1038/s42003-022-03351-7. Commun Biol. 2022. PMID: 35501403 Free PMC article.
-
Conformational flexibility of His200 enables catalytic activity in the T200H mutant of carbonic anhydrase II.Mol Cells. 2025 Jul;48(7):100226. doi: 10.1016/j.mocell.2025.100226. Epub 2025 May 27. Mol Cells. 2025. PMID: 40441537 Free PMC article.
-
2-Aminobenzoxazole-appended coumarins as potent and selective inhibitors of tumour-associated carbonic anhydrases.J Enzyme Inhib Med Chem. 2022 Dec;37(1):168-177. doi: 10.1080/14756366.2021.1998026. J Enzyme Inhib Med Chem. 2022. PMID: 34894971 Free PMC article.
References
-
- Lippard SJ, Berg JM. Principles of Bioinorganic Chemistry. CA: University Science Books Mill Valley; 1994.
-
- Holm RH, Kennepohl P, Solomon EI. Structural and functional aspects of metal sites in biology. Chem. Rev. 1996;96:2239–2314. - PubMed
-
- Ragsdale SW. Metals and their scaffolds to promote difficult enzymatic reactions. Chem. Rev. 2006;106:3317–3337. - PubMed
-
- Rulisek L, Vondrasek J. Coordination geometries of selected transition metal ions (Co2+, Ni2+, Cu2+, Zn2+, Cd2+, and Hg2+) in metalloproteins. J. Inorg. Biochem. 1998;71:115–127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

