A flavin-dependent monooxygenase catalyzes the initial step in cyanogenic glycoside synthesis in ferns
- PMID: 32917937
- PMCID: PMC7486406
- DOI: 10.1038/s42003-020-01224-5
A flavin-dependent monooxygenase catalyzes the initial step in cyanogenic glycoside synthesis in ferns
Abstract
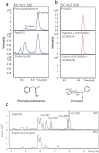
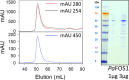
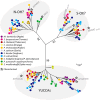
Cyanogenic glycosides form part of a binary plant defense system that, upon catabolism, detonates a toxic hydrogen cyanide bomb. In seed plants, the initial step of cyanogenic glycoside biosynthesis-the conversion of an amino acid to the corresponding aldoxime-is catalyzed by a cytochrome P450 from the CYP79 family. An evolutionary conundrum arises, as no CYP79s have been identified in ferns, despite cyanogenic glycoside occurrence in several fern species. Here, we report that a flavin-dependent monooxygenase (fern oxime synthase; FOS1), catalyzes the first step of cyanogenic glycoside biosynthesis in two fern species (Phlebodium aureum and Pteridium aquilinum), demonstrating convergent evolution of biosynthesis across the plant kingdom. The FOS1 sequence from the two species is near identical (98%), despite diversifying 140 MYA. Recombinant FOS1 was isolated as a catalytic active dimer, and in planta, catalyzes formation of an N-hydroxylated primary amino acid; a class of metabolite not previously observed in plants.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Harper, N. L., Cooper-Driver, G. A. & Swain, T. A survey for cyanogenesis in ferns and gymnosperms. Phytochemistry15, 1764–1767 (1976).
-
- Gleadow, R. M. & Møller, B. L. Cyanogenic glycosides: synthesis, physiology, and phenotypic plasticity. Ann. Rev. Plant Biol.65, 155–185 (2014). - PubMed
-
- Sanchez-Perez, R. et al. Mutation of a bHLH transcription factor allowed almond domestication. Science364, 1095–1098 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

