A single-cell polony method reveals low levels of infected Prochlorococcus in oligotrophic waters despite high cyanophage abundances
- PMID: 32918065
- PMCID: PMC7853090
- DOI: 10.1038/s41396-020-00752-6
A single-cell polony method reveals low levels of infected Prochlorococcus in oligotrophic waters despite high cyanophage abundances
Abstract
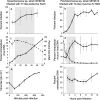
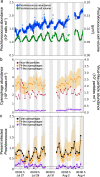
Long-term stability of picocyanobacteria in the open oceans is maintained by a balance between synchronous division and death on daily timescales. Viruses are considered a major source of microbial mortality, however, current methods to measure infection have significant methodological limitations. Here we describe a method that pairs flow-cytometric sorting with a PCR-based polony technique to simultaneously screen thousands of taxonomically resolved individual cells for intracellular virus DNA, enabling sensitive, high-throughput, and direct quantification of infection by different virus lineages. Under controlled conditions with picocyanobacteria-cyanophage models, the method detected infection throughout the lytic cycle and discriminated between varying infection levels. In North Pacific subtropical surface waters, the method revealed that only a small percentage of Prochlorococcus (0.35-1.6%) were infected, predominantly by T4-like cyanophages, and that infection oscillated 2-fold in phase with the diel cycle. This corresponds to 0.35-4.8% of Prochlorococcus mortality daily. Cyanophages were 2-4-fold more abundant than Prochlorococcus, indicating that most encounters did not result in infection and suggesting infection is mitigated via host resistance, reduced phage infectivity and inefficient adsorption. This method will enable quantification of infection for key microbial taxa across oceanic regimes and will help determine the extent that viruses shape microbial communities and ecosystem level processes.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Zwirglmaier K, Jardillier L, Ostrowski M, Mazard S, Garczarek L, Vaulot D, et al. Global phylogeography of marine Synechococcus and Prochlorococcus reveals a distinct partitioning of lineages among oceanic biomes. Environ Microbiol. 2008;10:147–61. - PubMed
-
- Malmstrom RR, Coe A, Kettler GC, Martiny AC, Frias-Lopez J, Zinser ER, et al. Temporal dynamics of Prochlorococcus ecotypes in the Atlantic and Pacific oceans. ISME J. 2010;4:1252–64. - PubMed
-
- Rii YM, Karl DM, Church MJ. Temporal and vertical variability in picophytoplankton primary productivity in the North Pacific Subtropical Gyre. Mar Ecol Prog Ser. 2016;562:1–18.
-
- Liu H, Nolla HA, Campbell L. Prochlorococcus growth rate and contribution to primary production in the equatorial and subtropical North Pacific Ocean. Aquat Micro Ecol. 1997;12:39–47.
-
- Vaulot D, Marie D, Olson RJ, Chisholm SW. Growth of Prochlorococcus, a photosynthetic prokaryote, in the Equatorial Pacific. Ocean Sci. 1995;268:1480–2. - PubMed

