Loss of anti-Müllerian hormone (AMH) immunoactivity due to a homozygous AMH gene variant rs10417628 in a woman with classical polycystic ovary syndrome (PCOS)
- PMID: 32918081
- PMCID: PMC7518710
- DOI: 10.1093/humrep/deaa199
Loss of anti-Müllerian hormone (AMH) immunoactivity due to a homozygous AMH gene variant rs10417628 in a woman with classical polycystic ovary syndrome (PCOS)
Abstract
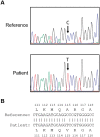
Anti-Müllerian hormone (AMH) is produced by granulosa cells of pre-antral and small antral ovarian follicles. In polycystic ovary syndrome (PCOS), higher levels of serum AMH are usually encountered due to the ample presence of small antral follicles and a high AMH production per follicular unit which have led to the proposal of AMH as a serum diagnostic marker for PCOS or as a surrogate for polycystic ovarian morphology (PCOM). However, heterozygous coding mutations of the AMH gene with decreased in vitro bioactivity have been described in some women with PCOS. Such mutation carriers have a trend toward reduced serum AMH levels compared to noncarriers, although both types of women with PCOS have similar circulating gonadotropin and testosterone (T) levels. This report describes a normal-weight woman with PCOS by NIH criteria with severely reduced AMH levels (index woman with PCOS). Our objective was to examine the molecular basis for her reduced serum AMH levels and to compare her endocrine characteristics to similar-weight women with PCOS and detectable AMH levels. Twenty normoandrogenic ovulatory (control) and 13 age- and BMI-matched women with PCOS (19-35 years; 19-25 kg/m2) underwent transvaginal sonography and serum hormone measures including gonadotropins, sex hormone-binding globulin, total and free T, androstenedione, dehydroepiandrosterone sulfate, estrone, estradiol and AMH. The latter was measured by ELISA (Pico-AMH: Ansh Labs, Webster, TX, USA). Women with PCOS and detectable AMH had higher serum AMH (10.82 (6.74-13.40) ng/ml, median (interquartile range)), total and free T (total T: 55.5 (49.5-62.5) ng/dl; free T: 5.65 (4.75-6.6) pg/ml) levels and greater total antral follicle count (AFC) (46 (39-59) follicles) than controls (AMH: 4.03 (2.47-6.11) ng/ml; total T: 30 (24.5-34.5) ng/dl; free T: 2.2 (1.8-2.45) pg/ml; AFC 16 (14.5-21.5) follicles, P < 0.05, all values), along with a trend toward LH hypersecretion (P = 0.06). The index woman with PCOS had severely reduced serum AMH levels (∼0.1 ng/ml), although she also had a typical NIH-defined PCOS phenotype resembling that of the other women with PCOS and elevated AMH levels. All women with PCOS, including the index woman with PCOS, exhibited LH hypersecretion, hyperandrogenism, reduced serum estrogen/androgen ratios and PCOM. A homozygous Ala515Val variant (rs10417628) in the mature region of AMH was identified in the index woman with PCOS. Recombinant hAMH-515Val displayed normal processing and bioactivity, yet had severely reduced immunoactivity when measured by the commercial pico-AMH ELISA assay by Ansh Labs. In conclusion, homozygous AMH variant rs10417628 may severely impair serum AMH immunoactivity without affecting its bioactivity or PCOS phenotypic expression. Variants in AMH can interfere with serum AMH immunoactivity without affecting the phenotype in PCOS. This observation can be accompanied by discordance between AMH immunoactivity and bioactivity.
Keywords: anti-Müllerian hormone; gene mutations; hyperandrogenism; polycystic ovary syndrome; variants.
© The Author(s) 2020. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Andersen CY, Byskov AG.. Estradiol and regulation of anti-Mullerian hormone, inhibin-A, and inhibin-B secretion: analysis of small antral and preovulatory human follicles’ fluid. J Clin Endocrinol Metab 2006;91:4064–4069. - PubMed
-
- Broekmans FJ, Visser JA, Laven JS, Broer SL, Themmen AP, Fauser BC.. Anti-Mullerian hormone and ovarian dysfunction. Trends Endocrinol Metab 2008;19:340–347. - PubMed
-
- Campbell BK, Clinton M, Webb R.. The role of anti-Mullerian hormone (AMH) during follicle development in a monovulatory species (sheep). Endocrinology 2012;153:4533–4543. - PubMed
-
- Cate RL, Mattaliano RJ, Hession C, Tizard R, Farber NM, Cheung A, Ninfa EG, Frey AZ, Gash DJ, Chow EP. et al. Isolation of the bovine and human genes for Mullerian inhibiting substance and expression of the human gene in animal cells. Cell 1986;45:685–698. - PubMed
-
- Catteau-Jonard S, Dewailly D.. Pathophysiology of polycystic ovary syndrome: the role of hyperandrogenism. Front Horm Res 2013;40:22–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

