Mortality and Disease Severity Among COVID-19 Patients Receiving Renin-Angiotensin System Inhibitors: A Systematic Review and Meta-analysis
- PMID: 32918209
- PMCID: PMC7486167
- DOI: 10.1007/s40256-020-00439-5
Mortality and Disease Severity Among COVID-19 Patients Receiving Renin-Angiotensin System Inhibitors: A Systematic Review and Meta-analysis
Abstract
Introduction: The use of renin-angiotensin system (RAS) inhibitors, including angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), was alleged to cause a more severe course of novel coronavirus disease 2019 (COVID-19).
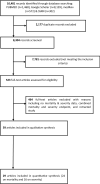
Methods: We systematically reviewed the published studies to assess the association of RAS inhibitors with mortality as well as disease severity in COVID-19 patients. A systematic literature search was performed to retrieve relevant original studies investigating mortality and severity (severe/critical disease) in COVID-19 patients with and without exposure to RAS inhibitors.
Results: A total of 59 original studies were included for qualitative synthesis. Twenty-four studies that reported adjusted effect sizes (24 studies reported mortality outcomes and 16 studies reported disease severity outcomes), conducted in RAS inhibitor-exposed and unexposed groups, were pooled in random-effects models to estimate overall risk. Quality assessment of studies revealed that most of the studies included were of fair quality. The use of an ACEI/ARB in COVID-19 patients was significantly associated with lower odds (odds ratio [OR] = 0.73, 95% confidence interval [CI] 0.56-0.95; n = 18,749) or hazard (hazard ratio [HR] = 0.75, 95% CI 0.60-0.95; n = 26,598) of mortality compared with non-use of ACEI/ARB. However, the use of an ACEI/ARB was non-significantly associated with lower odds (OR = 0.91, 95% CI 0.75-1.10; n = 7446) or hazard (HR = 0.73, 95% CI 0.33-1.66; n = 6325) of developing severe/critical disease compared with non-use of an ACEI/ARB.
Discussion: Since there was no increased risk of harm, the use of RAS inhibitors for hypertension and other established clinical indications can be maintained in COVID-19 patients.
Conflict of interest statement
Syed Shahzad Hasan, Chia Siang Kow, Muhammad Abdul Hadi, Syed Tabish Razi Zaidi, and Hamid A. Merchant declare that they have no potential conflicts of interest that might be relevant to the contents of this article.
Figures


References
-
- Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. - PubMed
-
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published correction appears in Lancet. 2020 Mar 28;395(10229):1038] [published correction appears in Lancet. 2020 Mar 28;395(10229):1038]. Lancet. 2020;395(10229):1054–1062. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

