The Role of Classical and Novel Forms of Vitamin D in the Pathogenesis and Progression of Nonmelanoma Skin Cancers
- PMID: 32918223
- PMCID: PMC7490773
- DOI: 10.1007/978-3-030-46227-7_13
The Role of Classical and Novel Forms of Vitamin D in the Pathogenesis and Progression of Nonmelanoma Skin Cancers
Abstract
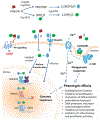
Nonmelanoma skin cancers including basal and squamous cell carcinomas (SCC and BCC) represent a significant clinical problem due to their relatively high incidence, imposing an economic burden to healthcare systems around the world. It is accepted that ultraviolet radiation (UVR: λ = 290-400 nm) plays a crucial role in the initiation and promotion of BCC and SCC with UVB (λ = 290-320 nm) having a central role in this process. On the other hand, UVB is required for vitamin D3 (D3) production in the skin, which supplies >90% of the body's requirement for this prohormone. Prolonged exposure to UVB can also generate tachysterol and lumisterol. Vitamin D3 itself and its canonical (1,25(OH)2D3) and noncanonical (CYP11A1-intitated) D3 hydroxyderivatives show photoprotective functions in the skin. These include regulation of keratinocyte proliferation and differentiation, induction of anti-oxidative responses, inhibition of DNA damage and induction of DNA repair mechanisms, and anti-inflammatory activities. Studies in animals have demonstrated that D3 hydroxyderivatives can attenuate UVB or chemically induced epidermal cancerogenesis and inhibit growth of SCC and BCC. Genomic and non-genomic mechanisms of action have been suggested. In addition, vitamin D3 itself inhibits hedgehog signaling pathways which have been implicated in many cancers. Silencing of the vitamin D receptor leads to increased propensity to develop UVB or chemically induced epidermal cancers. Other targets for vitamin D compounds include 1,25D3-MARRS, retinoic orphan receptors α and γ, aryl hydrocarbon receptor, and Wnt signaling. Most recently, photoprotective effects of lumisterol hydroxyderivatives have been identified. Clinical trials demonstrated a beneficial role of vitamin D compounds in the treatment of actinic keratosis. In summary, recent advances in vitamin D biology and pharmacology open new exciting opportunities in chemoprevention and treatment of skin cancers.
Keywords: Basal cell carcinoma; RORα; RORγ; Squamous cell carcinoma; Ultraviolet radiation; VDR; Vitamin D.
Figures




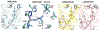

Similar articles
-
Photoprotective Properties of Vitamin D and Lumisterol Hydroxyderivatives.Cell Biochem Biophys. 2020 Jun;78(2):165-180. doi: 10.1007/s12013-020-00913-6. Epub 2020 May 22. Cell Biochem Biophys. 2020. PMID: 32441029 Free PMC article. Review.
-
CYP11A1‑derived vitamin D hydroxyderivatives as candidates for therapy of basal and squamous cell carcinomas.Int J Oncol. 2022 Aug;61(2):96. doi: 10.3892/ijo.2022.5386. Epub 2022 Jul 1. Int J Oncol. 2022. PMID: 35775377 Free PMC article.
-
The Impact of Vitamin D on Skin Aging.Int J Mol Sci. 2021 Aug 23;22(16):9097. doi: 10.3390/ijms22169097. Int J Mol Sci. 2021. PMID: 34445803 Free PMC article. Review.
-
On the role of classical and novel forms of vitamin D in melanoma progression and management.J Steroid Biochem Mol Biol. 2018 Mar;177:159-170. doi: 10.1016/j.jsbmb.2017.06.013. Epub 2017 Jul 1. J Steroid Biochem Mol Biol. 2018. PMID: 28676457 Free PMC article. Review.
-
Vitamin D signaling and melanoma: role of vitamin D and its receptors in melanoma progression and management.Lab Invest. 2017 Jun;97(6):706-724. doi: 10.1038/labinvest.2017.3. Epub 2017 Feb 20. Lab Invest. 2017. PMID: 28218743 Free PMC article. Review.
Cited by
-
Vitamin D Receptor Regulates the Expression of the Grainyhead-Like 1 Gene.Int J Mol Sci. 2024 Jul 19;25(14):7913. doi: 10.3390/ijms25147913. Int J Mol Sci. 2024. PMID: 39063155 Free PMC article.
-
Effects of Different Routes and Forms of Vitamin D Administration on Mesenteric Lymph Node CD4+ T Cell Polarization and Intestinal Injury in Obese Mice Complicated with Polymicrobial Sepsis.Nutrients. 2022 Aug 29;14(17):3557. doi: 10.3390/nu14173557. Nutrients. 2022. PMID: 36079813 Free PMC article.
-
Environmental Air Pollutants Affecting Skin Functions with Systemic Implications.Int J Mol Sci. 2023 Jun 22;24(13):10502. doi: 10.3390/ijms241310502. Int J Mol Sci. 2023. PMID: 37445680 Free PMC article. Review.
-
Photoprotective Properties of Vitamin D and Lumisterol Hydroxyderivatives.Cell Biochem Biophys. 2020 Jun;78(2):165-180. doi: 10.1007/s12013-020-00913-6. Epub 2020 May 22. Cell Biochem Biophys. 2020. PMID: 32441029 Free PMC article. Review.
-
Evolutionary formation of melatonin and vitamin D in early life forms: insects take centre stage.Biol Rev Camb Philos Soc. 2024 Oct;99(5):1772-1790. doi: 10.1111/brv.13091. Epub 2024 Apr 30. Biol Rev Camb Philos Soc. 2024. PMID: 38686544 Review.
References
-
- Addison CC, Gamlen GA, Thompson R. The ultraviolet absorption spectra of sodium hyponitrite and sodium α-oxyhyponitrite: the analysis of mixtures with sodium nitrite and nitrate. J Chem Soc. 1952:338–45.
-
- Ahmad N, Mukhtar H. Cytochrome p450: a target for drug development for skin diseases. J Invest Dermatol. 2004;123:417–25. - PubMed
-
- Albert B, Hahn H. Interaction of hedgehog and vitamin D signaling pathways in basal cell carcinomas. Adv Exp Med Biol. 2014;810:329–41. - PubMed
-
- American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, American Society For Mohs Surgery, Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, Fazio MJ, Storrs PA, Vidimos AT, Zalla MJ, Brewer JD, Begolka WS, Berger TG, Bigby M, Bolognia JL, Brodland DG, Collins S, Cronin TA Jr, Dahl MV, Grant-Kels JM, Hanke CW, Hruza GJ, James WD, Lober CW, Mcburney EI, Norton SA, Roenigk RK, Wheeland RG, Wisco OJ. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582–603. - PubMed
-
- Annalora AJ, Jozic M, Marcus CB, Iversen PL. Alternative splicing of the vitamin D receptor modulates target gene expression and promotes ligand-independent functions. Toxicol Appl Pharmacol. 2019;364:55–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

