A circulating exosomal microRNA panel as a novel biomarker for monitoring post-transplant renal graft function
- PMID: 32918330
- PMCID: PMC7579686
- DOI: 10.1111/jcmm.15861
A circulating exosomal microRNA panel as a novel biomarker for monitoring post-transplant renal graft function
Abstract
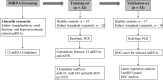
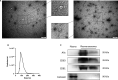
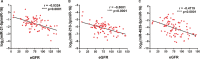
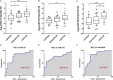
Accurate and effective biomarkers for continuous monitoring of graft function are needed after kidney transplantation. The aim of this study was to establish a circulating exosomal miRNA panel as non-invasive biomarker for kidney transplant recipients. Plasma exosomes of 58 kidney transplant recipients and 27 healthy controls were extracted by gel exclusion chromatography and characterized by transmission electron microscopy, nanoparticle tracking analysis and Western blotting. Post-transplant renal graft function was evaluated by estimated glomerular filtration rate (eGFR). Quantitative real-time polymerase chain reaction was used to determine the expression of exosomal microRNAs (miRNAs). Exosomal miR-21, miR-210 and miR-4639 showed negative correlations with eGFR in the training set and were selected for further analysis. In the validation set, miR-21, miR-210 and miR-4639 showed the capability to discriminate between subjects with chronic allograft dysfunction (eGFR < 60 mL/min/1.73 m2 ) and those with normal graft function (eGFR > 90 mL/min/1.73 m2 ). Three-miRNA panel exhibited higher accuracy compared with individual miRNAs or double indicators. One-year follow-up revealed a stable recovery of allograft function in subjects with low calculated score from three-miRNA panel (below the optimal cut-off value). In conclusion, a unique circulating exosomal miRNA panel was identified as an effective biomarker for monitoring post-transplant renal graft function in this study.
Keywords: MicroRNAs; biomarker; estimated glomerular filtration rate; exosomes; kidney transplant.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest. The authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and the accuracy of the data analysis.
Figures
References
-
- Leeson S, Desai SP. Medical and ethical challenges during the first successful human kidney transplantation in 1954 at Peter Bent Brigham Hospital, Boston. Anesth Analg. 2015;120:239‐245. - PubMed
-
- Schauerte C, Hubner A, Rong S, et al. Antagonism of profibrotic microRNA‐21 improves outcome of murine chronic renal allograft dysfunction. Kidney Int. 2017;92:646‐656. - PubMed
-
- Zununi Vahed S, Poursadegh Zonouzi A, Mahmoodpoor F, et al. Circulating miR‐150, miR‐192, miR‐200b, and miR‐423‐3p as non‐invasive biomarkers of chronic allograft dysfunction. Arch Med Res. 2017;48:96‐104. - PubMed
-
- Mannon RB, Kirk AD. Beyond histology: novel tools to diagnose allograft dysfunction. Clin J Am Soc Nephrol. 2006;1:358‐366. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

