Doxorubicin cardiomyopathy is ameliorated by acacetin via Sirt1-mediated activation of AMPK/Nrf2 signal molecules
- PMID: 32918384
- PMCID: PMC7579684
- DOI: 10.1111/jcmm.15859
Doxorubicin cardiomyopathy is ameliorated by acacetin via Sirt1-mediated activation of AMPK/Nrf2 signal molecules
Abstract
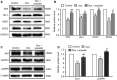
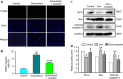
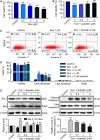
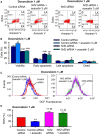
Doxorubicin cardiotoxicity is frequently reported in patients undergoing chemotherapy. The present study investigates whether cardiomyopathy induced by doxorubicin can be improved by the natural flavone acacetin in a mouse model and uncovers the potential molecular mechanism using cultured rat cardiomyoblasts. It was found that the cardiac dysfunction and myocardial fibrosis induced by doxorubicin were significantly improved by acacetin in mice with impaired Nrf2/HO-1 and Sirt1/pAMPK molecules, which is reversed by acacetin treatment. Doxorubicin decreased cell viability and increased ROS production in rat cardiomyoblasts; these effects are significantly countered by acacetin (0.3-3 μM) in a concentration-dependent manner via activating Sirt1/pAMPK signals and enhancing antioxidation (Nrf2/HO-1 and SOD1/SOD2) and anti-apoptosis. These protective effects were abolished in cells with silencing Sirt1. The results demonstrate for the first time that doxorubicin cardiotoxicity is antagonized by acacetin via Sirt1-mediated activation of AMPK/Nrf2 signal molecules, indicating that acacetin may be a drug candidate used clinically for protecting against doxorubicin cardiomyopathy.
Keywords: Sirt1; acacetin; antioxidant; cardiotoxicity; doxorubicin; oxidative stress.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Acacetin ameliorates cardiac hypertrophy by activating Sirt1/AMPK/PGC-1α pathway.Eur J Pharmacol. 2022 Apr 5;920:174858. doi: 10.1016/j.ejphar.2022.174858. Epub 2022 Feb 24. Eur J Pharmacol. 2022. PMID: 35219729
-
Cardiac senescence is alleviated by the natural flavone acacetin via enhancing mitophagy.Aging (Albany NY). 2021 Jun 27;13(12):16381-16403. doi: 10.18632/aging.203163. Epub 2021 Jun 27. Aging (Albany NY). 2021. PMID: 34175838 Free PMC article.
-
Melatonin alleviates doxorubicin-induced cardiotoxicity via inhibiting oxidative stress, pyroptosis and apoptosis by activating Sirt1/Nrf2 pathway.Biomed Pharmacother. 2023 Jun;162:114591. doi: 10.1016/j.biopha.2023.114591. Epub 2023 Mar 23. Biomed Pharmacother. 2023. PMID: 36965257
-
Acacetin is a Promising Drug Candidate for Cardiovascular Diseases.Am J Chin Med. 2024;52(6):1661-1692. doi: 10.1142/S0192415X24500654. Epub 2024 Sep 30. Am J Chin Med. 2024. PMID: 39347953 Review.
-
The modulation of SIRT1 and SIRT3 by natural compounds as a therapeutic target in doxorubicin-induced cardiotoxicity: A review.J Biochem Mol Toxicol. 2022 Jan;36(1):e22946. doi: 10.1002/jbt.22946. Epub 2021 Nov 8. J Biochem Mol Toxicol. 2022. PMID: 34747550 Review.
Cited by
-
Sirt1 Overexpression Inhibits Fibrous Scar Formation and Improves Functional Recovery After Cerebral Ischemic Injury Through the Deacetylation of 14-3-3ζ.Mol Neurobiol. 2023 Aug;60(8):4795-4810. doi: 10.1007/s12035-023-03378-9. Epub 2023 May 10. Mol Neurobiol. 2023. PMID: 37162725
-
Isoquercitrin Suppresses Esophageal Squamous Cell Carcinoma (ESCC) by Inducing Excessive Autophagy and Promoting Apoptosis via the AKT/mTOR Signaling Pathway.Antioxidants (Basel). 2025 Jun 8;14(6):694. doi: 10.3390/antiox14060694. Antioxidants (Basel). 2025. PMID: 40563327 Free PMC article.
-
NMDARs antagonist MK801 suppresses LPS-induced apoptosis and mitochondrial dysfunction by regulating subunits of NMDARs via the CaM/CaMKII/ERK pathway.Cell Death Discov. 2023 Feb 11;9(1):59. doi: 10.1038/s41420-023-01362-9. Cell Death Discov. 2023. PMID: 36774369 Free PMC article.
-
Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns.Molecules. 2021 Jun 30;26(13):4021. doi: 10.3390/molecules26134021. Molecules. 2021. PMID: 34209338 Free PMC article. Review.
-
A comprehensive account on ethnobotany, phytochemistry and pharmacological insights of genus Celtis.Heliyon. 2024 Apr 25;10(9):e29707. doi: 10.1016/j.heliyon.2024.e29707. eCollection 2024 May 15. Heliyon. 2024. PMID: 38726115 Free PMC article. Review.
References
-
- Takemura G, Fujiwara H. Doxorubicin‐induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49:330‐352. - PubMed
-
- Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin‐induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52:1213‐1225. - PubMed
-
- Singal PK, Iliskovic N. Doxorubicin‐induced cardiomyopathy. N Engl J Med. 1998;339:900‐905. - PubMed
-
- Vejpongsa P, Yeh ET. Prevention of anthracycline‐induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol. 2014;64:938‐945. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

