Cancer cachexia in a mouse model of oxidative stress
- PMID: 32918528
- PMCID: PMC7749559
- DOI: 10.1002/jcsm.12615
Cancer cachexia in a mouse model of oxidative stress
Abstract
Background: Cancer is associated with muscle atrophy (cancer cachexia) that is linked to up to 40% of cancer-related deaths. Oxidative stress is a critical player in the induction and progression of age-related loss of muscle mass and weakness (sarcopenia); however, the role of oxidative stress in cancer cachexia has not been defined. The purpose of this study was to examine if elevated oxidative stress exacerbates cancer cachexia.
Methods: Cu/Zn superoxide dismutase knockout (Sod1KO) mice were used as an established mouse model of elevated oxidative stress. Cancer cachexia was induced by injection of one million Lewis lung carcinoma (LLC) cells or phosphate-buffered saline (saline) into the hind flank of female wild-type mice or Sod1KO mice at approximately 4 months of age. The tumour developed for 3 weeks. Muscle mass, contractile function, neuromuscular junction (NMJ) fragmentation, metabolic proteins, mitochondrial function, and motor neuron function were measured in wild-type and Sod1KO saline and tumour-bearing mice. Data were analysed by two-way ANOVA with Tukey-Kramer post hoc test when significant F ratios were determined and α was set at 0.05. Unless otherwise noted, results in abstract are mean ±SEM.
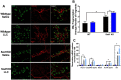
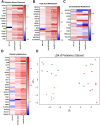
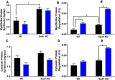
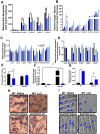
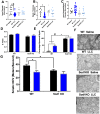
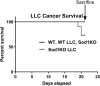
Results: Muscle mass and cross-sectional area were significantly reduced, in tumour-bearing mice. Metabolic enzymes were dysregulated in Sod1KO mice and cancer exacerbated this phenotype. NMJ fragmentation was exacerbated in tumour-bearing Sod1KO mice. Myofibrillar protein degradation increased in tumour-bearing wild-type mice (wild-type saline, 0.00847 ± 0.00205; wildtype LLC, 0.0211 ± 0.00184) and tumour-bearing Sod1KO mice (Sod1KO saline, 0.0180 ± 0.00118; Sod1KO LLC, 0.0490 ± 0.00132). Muscle mitochondrial oxygen consumption was reduced in tumour-bearing mice compared with saline-injected wild-type mice. Mitochondrial protein degradation increased in tumour-bearing wild-type mice (wild-type saline, 0.0204 ± 0.00159; wild-type LLC, 0.167 ± 0.00157) and tumour-bearing Sod1KO mice (Sod1KO saline, 0.0231 ± 0.00108; Sod1 KO LLC, 0.0645 ± 0.000631). Sciatic nerve conduction velocity was decreased in tumour-bearing wild-type mice (wild-type saline, 38.2 ± 0.861; wild-type LLC, 28.8 ± 0.772). Three out of eleven of the tumour-bearing Sod1KO mice did not survive the 3-week period following tumour implantation.
Conclusions: Oxidative stress does not exacerbate cancer-induced muscle loss; however, cancer cachexia may accelerate NMJ disruption.
Keywords: CuZn superoxide dismutase knockout mice (Sod1KO); Lewis lung carcinoma cells (LLC); Lung cancer; Oxidative stress; Reactive oxygen species (ROS).
© 2020 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures


References
-
- Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell sMetab 2012;16:153–166. - PubMed
-
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. - PubMed
-
- Davis MP, Panikkar R. Sarcopenia associated with chemotherapy and targeted agents for cancer therapy. Ann Palliat Med 2019;8:86–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

