Accuracy of a RT-qPCR SARS-CoV-2 detection assay without prior RNA extraction
- PMID: 32918932
- PMCID: PMC7480275
- DOI: 10.1016/j.jviromet.2020.113969
Accuracy of a RT-qPCR SARS-CoV-2 detection assay without prior RNA extraction
Abstract
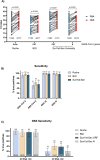
The current COVID-19 pandemic constitutes a threat to the population worldwide with over 21 million infected people. There is an urgent need for the development of rapid and massive detection tools as well as the identification and isolation of infected individuals. we sought to evaluate different RT-qPCR kits and protocols to evaluate the best approach to be used omitting an RNA extraction step. We have investigated the sensitivity and performance of different commercially available RT-qPCR kits in detecting SARS-CoV-2 using 80 extracted RNA and NSS from COVID-19 diagnosed patients. We evaluated the ability of each kit to detect viral RNA from both kit-extracted or directly from a pre-boiled NSS observing that direct RNA detection is possible when Ct values are lower than 30 with the three kits tested. Since SARS-CoV-2 testing in most locations occurs once COVID-19 symptoms are evident and, therefore, viral loads are expected to be high, our protocol will be useful in supporting SARS-CoV-2 diagnosis, especially in America where COVID-19 cases have exploded in the recent weeks as well as in low- and middle-income countries, which would not have massive access to kit-based diagnosis. The information provided in this work paves the way for the development of more efficient SARS-CoV-2 detection approaches avoiding an RNA extraction step.
Keywords: 2019-nCoV; COVID-19; RNA extraction; RT-PCR; SARS-CoV-2.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors report no declarations of interest.
Figures
References
-
- Beltrán-Pavez C., Márquez C., Muñoz G., Valiente-Echeverría F., Gaggero A., Soto-Rifo R., Barriga G. SARS-CoV-2 detection from nasopharyngeal swab samples without RNA extraction. bioRxiv. 2020 doi: 10.1101/2020.03.28.013508. 2020.03.28.013508. - DOI
-
- To K.K.W., Tsang O.T.Y., Leung W.S., Tam A.R., Wu T.C., Lung D.C., Yip C.C.Y., Cai J.P., Chan J.M.C., Chik T.S.H., Lau D.P.L., Choi C.Y.C., Chen L.L., Chan W.M., Chan K.H., Ip J.D., Ng A.C.K., Poon R.W.S., Luo C.T., Cheng V.C.C., Chan J.F.W., Hung I.F.N., Chen Z., Chen H., Yuen K.Y. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

