Maternal obesity causes fetal hypothalamic insulin resistance and disrupts development of hypothalamic feeding pathways
- PMID: 32919096
- PMCID: PMC7549144
- DOI: 10.1016/j.molmet.2020.101079
Maternal obesity causes fetal hypothalamic insulin resistance and disrupts development of hypothalamic feeding pathways
Abstract
Objective: Perinatal exposure to maternal obesity results in predisposition of offspring to develop obesity later in life. Increased weight gain in offspring exposed to maternal obesity is usually associated with hyperphagia, implicating altered central regulation of food intake as a cause. We aimed to define how maternal obesity impacts early development of the hypothalamus to program lasting dysfunction in feeding regulatory pathways.
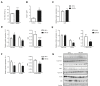
Methods: Mice offspring of diet-induced obese mothers were compared to the offspring of lean control mothers. We analysed gene expression in the fetal hypothalamus, alongside neurosphere assays to investigate the effects of maternal obesity on neural progenitor cell proliferation in vitro. Western blotting was used to investigate the insulin signalling pathway in the fetal hypothalamus. Characterisation of cell type and neuropeptide profile in adulthood was linked with analyses of feeding behaviour.
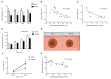
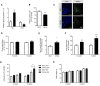
Results: There was a reduction in the expression of proliferative genes in the fetal hypothalamus of offspring exposed to maternal obesity. This reduction in proliferation was maintained in vitro when hypothalamic neural progenitor cells were grown as neurospheres. Hypothalamic fetal gene expression and neurosphere growth correlated with maternal body weight and insulin levels. Foetuses of obese mothers showed hypothalamic insulin resistance, which may be causative of reduced proliferation. Furthermore, maternal obesity activated the Notch signalling pathway in neonatal offspring hypothalamus, resulting in decreased neurogenesis. Adult offspring of obese mothers displayed an altered ratio of anorexigenic and orexigenic signals in the arcuate nucleus, associated with an inability to maintain energy homeostasis when metabolically challenged.
Conclusions: These findings show that maternal obesity alters the molecular signature in the developing hypothalamus, which is associated with disrupted growth and development of hypothalamic precursor cells and defective feeding regulation in adulthood. This is the first report of fetal hypothalamic insulin resistance in an obese pregnancy and suggests a mechanism by which maternal obesity causes permanent changes to hypothalamic structure and function.
Keywords: Hypothalamus; Insulin; Maternal obesity; Neurogenesis; Notch.
Copyright © 2020 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures

Similar articles
-
Female and male C57BL/6J offspring exposed to maternal obesogenic diet develop altered hypothalamic energy metabolism in adulthood.Am J Physiol Endocrinol Metab. 2022 Nov 1;323(5):E448-E466. doi: 10.1152/ajpendo.00100.2022. Epub 2022 Sep 21. Am J Physiol Endocrinol Metab. 2022. PMID: 36342228 Free PMC article.
-
Amylin receptor insensitivity impairs hypothalamic POMC neuron differentiation in the male offspring of maternal high-fat diet-fed mice.Mol Metab. 2021 Feb;44:101135. doi: 10.1016/j.molmet.2020.101135. Epub 2020 Dec 3. Mol Metab. 2021. PMID: 33279727 Free PMC article.
-
Maternal obesity increases hypothalamic miR-505-5p expression in mouse offspring leading to altered fatty acid sensing and increased intake of high-fat food.PLoS Biol. 2024 Jun 4;22(6):e3002641. doi: 10.1371/journal.pbio.3002641. eCollection 2024 Jun. PLoS Biol. 2024. PMID: 38833481 Free PMC article.
-
Mechanisms mediating the impact of maternal obesity on offspring hypothalamic development and later function.Front Endocrinol (Lausanne). 2022 Dec 22;13:1078955. doi: 10.3389/fendo.2022.1078955. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36619540 Free PMC article. Review.
-
Early life origins of metabolic disease: Developmental programming of hypothalamic pathways controlling energy homeostasis.Front Neuroendocrinol. 2015 Oct;39:3-16. doi: 10.1016/j.yfrne.2015.08.001. Epub 2015 Aug 19. Front Neuroendocrinol. 2015. PMID: 26296796 Review.
Cited by
-
Developmental programming of hypothalamic melanocortin circuits.Exp Mol Med. 2022 Apr;54(4):403-413. doi: 10.1038/s12276-021-00625-8. Epub 2022 Apr 26. Exp Mol Med. 2022. PMID: 35474338 Free PMC article. Review.
-
Update on Hypothalamic Inflammation and Gliosis: Expanding Evidence of Relevance Beyond Obesity.Curr Obes Rep. 2025 Jan 8;14(1):6. doi: 10.1007/s13679-024-00595-8. Curr Obes Rep. 2025. PMID: 39775194 Review.
-
Emerging role of hypothalamus in the metabolic regulation in the offspring of maternal obesity.Front Nutr. 2023 Feb 1;10:1094616. doi: 10.3389/fnut.2023.1094616. eCollection 2023. Front Nutr. 2023. PMID: 36819678 Free PMC article. Review.
-
Pre-pregnancy body mass index has greater influence on newborn weight and perinatal outcome than weight control during pregnancy in obese women.Arch Public Health. 2023 Jan 13;81(1):5. doi: 10.1186/s13690-023-01025-2. Arch Public Health. 2023. PMID: 36639806 Free PMC article.
-
Maternal pre-pregnancy body mass index is associated with newborn offspring hypothalamic mean diffusivity: a prospective dual-cohort study.BMC Med. 2023 Feb 14;21(1):57. doi: 10.1186/s12916-023-02743-8. BMC Med. 2023. PMID: 36788536 Free PMC article.
References
-
- Davies S. Department of Health; London: 2015. Annual report of the chief medical officer, 2014, the Health of the 51%: women.
-
- Perng W., Oken E., Dabelea D. Developmental overnutrition and obesity and type 2 diabetes in offspring. Diabetologia. 2019;62(10):1779–1788. - PubMed
-
- Smith J., Cianflone K., Biron S., Hould F.S., Lebel S., Marceau S. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. Journal of Clinical Endocrinology & Metabolism. 2009;94(11):4275–4283. - PubMed
-
- Dearden L., Ozanne S.E. Early life origins of metabolic disease: developmental programming of hypothalamic pathways controlling energy homeostasis. Frontiers in Neuroendocrinology. 2015;39:3–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

