Left prefrontal transcranial magnetic stimulation for treatment-resistant depression in adolescents: a double-blind, randomized, sham-controlled trial
- PMID: 32919400
- PMCID: PMC7852515
- DOI: 10.1038/s41386-020-00829-y
Left prefrontal transcranial magnetic stimulation for treatment-resistant depression in adolescents: a double-blind, randomized, sham-controlled trial
Abstract
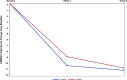
Treatment-resistant depression (TRD) is prevalent and associated with a substantial psychosocial burden and mortality. There are few prior studies of interventions for TRD in adolescents. This was the largest study to date examining the feasibility, safety, and efficacy of 10-Hz transcranial magnetic stimulation (TMS) for adolescents with TRD. Adolescents with TRD (aged 12-21 years) were enrolled in a randomized, sham-controlled trial of TMS across 13 sites. Treatment resistance was defined as an antidepressant treatment record level of 1 to 4 in a current episode of depression. Intention-to-treat patients (n = 103) included those randomly assigned to active NeuroStar TMS monotherapy (n = 48) or sham TMS (n = 55) for 30 daily treatments over 6 weeks. The primary outcome measure was change in the Hamilton Depression Rating Scale (HAM-D-24) score. After 6 weeks of blinded treatment, improvement in the least-squares mean (SE) HAM-D-24 scores were similar between the active (-11.1 [2.03]) and sham groups (-10.6 [2.00]; P = 0.8; difference [95% CI], - 0.5 [-4.2 to 3.3]). Response rates were 41.7% in the active group and 36.4% in the sham group (P = 0.6). Remission rates were 29.2% in the active group and 29.0% in the sham group (P = 0.95). There were no new tolerability or safety signals in adolescents. Although TMS treatment produced a clinically meaningful change in depressive symptom severity, this did not differ from sham treatment. Future studies should focus on strategies to reduce the placebo response and examine the optimal dosing of TMS for adolescents with TRD.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Brent D, Emslie G, Clarke G, Wagner KD, Asarnow JR, Keller M, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299:901–13. doi: 10.1001/jama.299.8.901. - DOI - PMC - PubMed
-
- March J, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA. 2004;292:807–20. doi: 10.1001/jama.292.7.807. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical