Empirical and theoretical insights into the structural effects of selenite doping in hydroxyapatite and the ensuing inhibition of osteoclasts
- PMID: 32919627
- PMCID: PMC7501993
- DOI: 10.1016/j.msec.2020.111257
Empirical and theoretical insights into the structural effects of selenite doping in hydroxyapatite and the ensuing inhibition of osteoclasts
Abstract
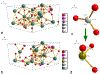
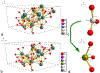
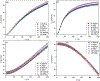
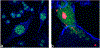
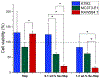
The use of ions as therapeutic agents has the potential to minimize the use of small-molecule drugs and biologics for the same purpose, thus providing a potentially more economic and less adverse means of treating, ameliorating or preventing a number of diseases. Hydroxyapatite (HAp) is a solid compound capable of accommodating foreign ions with a broad range of sizes and charges and its properties can dramatically change with the incorporation of these ionic additives. While most ionic substitutes in HAp have been monatomic cations, their lesser atomic weight, higher diffusivity, chaotropy and a lesser residence time on surfaces theoretically makes them prone to exert a lesser influence on the material/cell interaction than the more kosmotropic oxyanions. Selenite ion as an anionic substitution in HAp was explored in this study for its ability to affect the short-range and the long-range crystalline symmetry and solubility as well as for its ability to affect the osteoclast activity. We combined microstructural, crystallographic and spectroscopic analyses with quantum mechanical calculations to understand the structural effects of doping HAp with selenite. Integration of selenite ions into the crystal structure of HAp elongated the crystals along the c-axis, but isotropically lowered the crystallinity. It also increased the roughness of the material in direct proportion with the content of the selenite dopant, thus having a potentially positive effect on cell adhesion and integration with the host tissue. Selenite in total acted as a crystal structure breaker, but was also able to bring about symmetry at the local and global scales within specific concentration windows, indicating a variety of often mutually antagonistic crystallographic effects that it can induce in a concentration-dependent manner. Experimental determination of the lattice strain coupled with ab initio calculations on three different forms of carbonated HAp (A-type, B-type, AB-type) demonstrated that selenite ions initially substitute carbonates in the crystal structure of carbonated HAp, before substituting phosphates at higher concentrations. The most energetically favored selenite-doped HAp is of AB-type, followed by the B-type and only then by the A-type. This order of stability was entailed by the variation in the geometry and orientation of both the selenite ion and its neighboring phosphates and/or carbonates. The incorporation of selenite in different types of carbonated HAp also caused variations of different thermodynamic parameters, including entropy, enthalpy, heat capacity, and the Gibbs free energy. Solubility of HAp accommodating 1.2 wt% of selenite was 2.5 times higher than that of undoped HAp and the ensuing release of the selenite ion was directly responsible for inhibiting RAW264.7 osteoclasts. Dose-response curves demonstrated that the inhibition of osteoclasts was directly proportional to the concentration of selenite-doped HAp and to the selenite content in it. Meanwhile, selenite-doped HAp had a significantly less adverse effect on osteoblastic K7M2 and MC3T3-E1 cells than on RAW264.7 osteoclasts. The therapeutically promising osteoblast vs. osteoclast selectivity of inhibition was absent when the cells were challenged with undoped HAp, indicating that it is caused by selenite ions in HAp rather than by HAp alone. It is concluded that like three oxygens building the selenite pyramid, the coupling of (1) experimental materials science, (2) quantum mechanical modeling and (3) biological assaying is a triad from which a deeper understanding of ion-doped HAp and other biomaterials can emanate.
Keywords: Ab initio; Calcium phosphate; Hydroxyapatite; Nanoparticles; Osteoblasts; Selenite.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest There are no conflicts of interest to declare.
Figures
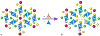

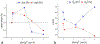

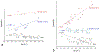



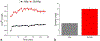
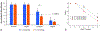
References
-
- Uskoković V, Ion-Doped Hydroxyapatite: An Impasse or the Road to Follow?, Ceram. Int. 46 (2020) 11443–11465.
-
- Mayer I, Berger U, Markitziu A, Gedalia I, The uptake of lithium ions by synthetic carbonated hydroxyapatite, Calcif. Tissue Int. 38 (1986) 293–295. - PubMed
-
- Arslan A, Cakmak S, Gumusderelioglu M, Enhanced osteogenic activity with boron-doped nanohydroxyapatite-loaded poly(butylene adipate-co-terephthalate) fibrous 3D matrix. Artificial Cells, Nanomedicine, and Biotechnology 46 (2018) 790–799. - PubMed
-
- Ciobanu G, Bargan AM, Luca C, New Bismuth-Substituted Hydroxyapatite Nanoparticles for Bone Tissue Engineering. JOM 67 (2015) 2534–42.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

