The phosphoinositide 3-kinase inhibitor alpelisib restores actin organization and improves proximal tubule dysfunction in vitro and in a mouse model of Lowe syndrome and Dent disease
- PMID: 32919786
- PMCID: PMC7550850
- DOI: 10.1016/j.kint.2020.05.040
The phosphoinositide 3-kinase inhibitor alpelisib restores actin organization and improves proximal tubule dysfunction in vitro and in a mouse model of Lowe syndrome and Dent disease
Abstract
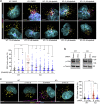
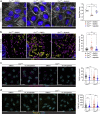
Loss-of-function mutations in the OCRL gene, which encodes the phosphatidylinositol [PI] 4,5-bisphosphate [PI(4,5)P2] 5-phosphatase OCRL, cause defective endocytosis and proximal tubule dysfunction in Lowe syndrome and Dent disease 2. The defect is due to increased levels of PI(4,5)P2 and aberrant actin polymerization, blocking endosomal trafficking. PI 3-phosphate [PI(3)P] has been recently identified as a coactivator with PI(4,5)P2 in the actin pathway. Here, we tested the hypothesis that phosphoinositide 3-kinase (PI3K) inhibitors may rescue the endocytic defect imparted by OCRL loss, by rebalancing phosphoinositide signals to the actin machinery. The broad-range PI3K inhibitor copanlisib and class IA p110α PI3K inhibitor alpelisib reduced aberrant actin polymerization in OCRL-deficient human kidney cells in vitro. Levels of PI 3,4,5-trisphosphate, PI(4,5)P2 and PI(3)P were all reduced with alpelisib treatment, and siRNA knockdown of the PI3K catalytic subunit p110α phenocopied the actin phenotype. In a humanized OcrlY/- mouse model, alpelisib reduced endosomal actin staining while restoring stress fiber architecture and levels of megalin at the plasma membrane of proximal tubule cells, reflected by improved endocytic uptake of low molecular weight proteins in vivo. Thus, our findings support the link between phosphoinositide lipids, actin polymerization and endocytic trafficking in the proximal tubule and represent a proof-of-concept for repurposing alpelisib in Lowe syndrome/Dent disease 2.
Keywords: cytoskeleton; endocytosis; lipids; proximal tubule; renal Fanconi syndrome.
Copyright © 2020 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Figures





Similar articles
-
OCRL deficiency impairs endolysosomal function in a humanized mouse model for Lowe syndrome and Dent disease.Hum Mol Genet. 2019 Jun 15;28(12):1931-1946. doi: 10.1093/hmg/ddy449. Hum Mol Genet. 2019. PMID: 30590522 Free PMC article.
-
OCRL-mutated fibroblasts from patients with Dent-2 disease exhibit INPP5B-independent phenotypic variability relatively to Lowe syndrome cells.Hum Mol Genet. 2015 Feb 15;24(4):994-1006. doi: 10.1093/hmg/ddu514. Epub 2014 Oct 9. Hum Mol Genet. 2015. PMID: 25305077
-
Loss of OCRL increases ciliary PI(4,5)P2 in Lowe oculocerebrorenal syndrome.J Cell Sci. 2017 Oct 15;130(20):3447-3454. doi: 10.1242/jcs.200857. Epub 2017 Sep 4. J Cell Sci. 2017. PMID: 28871046 Free PMC article.
-
The 5-phosphatase OCRL in Lowe syndrome and Dent disease 2.Nat Rev Nephrol. 2017 Aug;13(8):455-470. doi: 10.1038/nrneph.2017.83. Epub 2017 Jul 3. Nat Rev Nephrol. 2017. PMID: 28669993 Review.
-
The role of the Lowe syndrome protein OCRL in the endocytic pathway.Biol Chem. 2015 Dec;396(12):1293-300. doi: 10.1515/hsz-2015-0180. Biol Chem. 2015. PMID: 26351914 Review.
Cited by
-
Dent-2 disease with a Bartter-like phenotype caused by the Asp631Glu mutation in the OCRL gene.BMC Nephrol. 2022 May 12;23(1):182. doi: 10.1186/s12882-022-02812-9. BMC Nephrol. 2022. PMID: 35549682 Free PMC article.
-
Advances in Diagnosis and Treatment of Inherited Kidney Diseases in Children.Kidney Dis (Basel). 2024 Sep 24;10(6):558-572. doi: 10.1159/000541564. eCollection 2024 Dec. Kidney Dis (Basel). 2024. PMID: 39664340 Free PMC article. Review.
-
WHAMM functions in kidney reabsorption and polymerizes actin to promote autophagosomal membrane closure and cargo sequestration.Mol Biol Cell. 2024 Jun 1;35(6):ar80. doi: 10.1091/mbc.E24-01-0025. Epub 2024 Apr 10. Mol Biol Cell. 2024. PMID: 38598293 Free PMC article.
-
A 3D Renal Proximal Tubule on Chip Model Phenocopies Lowe Syndrome and Dent II Disease Tubulopathy.Int J Mol Sci. 2021 May 19;22(10):5361. doi: 10.3390/ijms22105361. Int J Mol Sci. 2021. PMID: 34069732 Free PMC article.
-
WHAMM functions in kidney reabsorption and polymerizes actin to promote autophagosomal membrane closure and cargo sequestration.bioRxiv [Preprint]. 2024 Jan 23:2024.01.22.576497. doi: 10.1101/2024.01.22.576497. bioRxiv. 2024. Update in: Mol Biol Cell. 2024 Jun 1;35(6):ar80. doi: 10.1091/mbc.E24-01-0025. PMID: 38328079 Free PMC article. Updated. Preprint.
References
-
- van der Wijst J., Belge H., Bindels R.J.M., Devuyst O. Learning physiology from inherited kidney disorders. Physiol Rev. 2019;99:1575–1653. - PubMed
-
- Lewis R.A., Nussbaum R.L., Brewer E.D. Lowe syndrome. In: Adam M.P., Ardinger H.H., Pagon R.A., editors. GeneReviews® [internet] University of Washington, Seattle; Seattle (WA): 1993-2020. https://www.ncbi.nlm.nih.gov/books/NBK1480/ Available at: Published July 24, 2001; Updated April 18, 2019. Accessed August 8, 2019.
-
- De Matteis M.A., Staiano L., Emma F., Devuyst O. The 5-phosphatase OCRL in Lowe syndrome and Dent disease 2. Nat Rev Nephrol. 2017;13:455–470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

