Keratocyte mechanobiology
- PMID: 32919993
- PMCID: PMC7655662
- DOI: 10.1016/j.exer.2020.108228
Keratocyte mechanobiology
Abstract
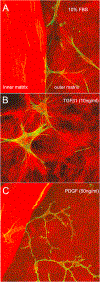
In vivo, corneal keratocytes reside within a complex 3D extracellular matrix (ECM) consisting of highly aligned collagen lamellae, growth factors, and other extracellular matrix components, and are subjected to various mechanical stimuli during developmental morphogenesis, fluctuations in intraocular pressure, and wound healing. The process by which keratocytes convert changes in mechanical stimuli (e.g. local topography, applied force, ECM stiffness) into biochemical signaling is known as mechanotransduction. Activation of the various mechanotransductive pathways can produce changes in cell migration, proliferation, and differentiation. Here we review how corneal keratocytes respond to and integrate different biochemical and biophysical factors. We first highlight how growth factors and other cytokines regulate the activity of Rho GTPases, cytoskeletal remodeling, and ultimately the mechanical phenotype of keratocytes. We then discuss how changes in the mechanical properties of the ECM have been shown to regulate keratocyte behavior in sophisticated 2D and 3D experimental models of the corneal microenvironment. Finally, we discuss how ECM topography and protein composition can modulate cell phenotypes, and review the different methods of fabricating in vitro mimics of corneal ECM topography, novel approaches for examining topographical effects in vivo, and the impact of different ECM glycoproteins and proteoglycans on keratocyte behavior.
Keywords: Cell mechanics; Corneal keratocytes; Corneal stroma; Extracellular matrix; Mechanobiology.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures





Similar articles
-
Mechanical interactions and crosstalk between corneal keratocytes and the extracellular matrix.Exp Eye Res. 2015 Apr;133:49-57. doi: 10.1016/j.exer.2014.09.003. Exp Eye Res. 2015. PMID: 25819454 Free PMC article. Review.
-
Growth factor regulation of corneal keratocyte mechanical phenotypes in 3-D collagen matrices.Invest Ophthalmol Vis Sci. 2012 Mar 1;53(3):1077-86. doi: 10.1167/iovs.11-8609. Print 2012 Mar. Invest Ophthalmol Vis Sci. 2012. PMID: 22247479 Free PMC article.
-
ECM stiffness modulates the proliferation but not the motility of primary corneal keratocytes in response to PDGF-BB.Exp Eye Res. 2022 Jul;220:109112. doi: 10.1016/j.exer.2022.109112. Epub 2022 May 18. Exp Eye Res. 2022. PMID: 35595094 Free PMC article.
-
MMP regulation of corneal keratocyte motility and mechanics in 3-D collagen matrices.Exp Eye Res. 2014 Apr;121:147-60. doi: 10.1016/j.exer.2014.02.002. Epub 2014 Feb 14. Exp Eye Res. 2014. PMID: 24530619 Free PMC article.
-
Morphological analysis of quiescent and activated keratocytes: a review of ex vivo and in vivo findings.Curr Eye Res. 2014 Dec;39(12):1129-44. doi: 10.3109/02713683.2014.902073. Epub 2014 Apr 21. Curr Eye Res. 2014. PMID: 24749788 Review.
Cited by
-
Advances in Regulatory Strategies of Differentiating Stem Cells towards Keratocytes.Stem Cells Int. 2022 Jan 31;2022:5403995. doi: 10.1155/2022/5403995. eCollection 2022. Stem Cells Int. 2022. PMID: 35140792 Free PMC article. Review.
-
Corneal Keratocytes, Fibroblasts, and Myofibroblasts Exhibit Distinct Transcriptional Profiles In Vitro.Invest Ophthalmol Vis Sci. 2025 Mar 3;66(3):28. doi: 10.1167/iovs.66.3.28. Invest Ophthalmol Vis Sci. 2025. PMID: 40072446 Free PMC article.
-
Stiffness promotes cell migration, invasion, and invadopodia in nasopharyngeal carcinoma by regulating the WT-CTTN level.Cancer Sci. 2024 Mar;115(3):836-846. doi: 10.1111/cas.16075. Epub 2024 Jan 26. Cancer Sci. 2024. PMID: 38273817 Free PMC article.
-
Collagen Crosslinking for Keratoconus: Cellular Signaling Mechanisms.Biomolecules. 2023 Apr 20;13(4):696. doi: 10.3390/biom13040696. Biomolecules. 2023. PMID: 37189443 Free PMC article.
-
Growth factors and mechano-regulated reciprocal crosstalk with extracellular matrix tune the keratocyte-fibroblast/myofibroblast transition.Sci Rep. 2023 Jul 13;13(1):11350. doi: 10.1038/s41598-023-37776-9. Sci Rep. 2023. PMID: 37443325 Free PMC article.
References
-
- Alberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, Walter P, 2015. Molecular biology of the cell, 6th ed. Garland Science, New York.
-
- Ali NQ, Patel DV, McGhee CN, 2014. Biomechanical responses of healthy and keratoconic corneas measured using a noncontact scheimpflug-based tonometer. Invest Ophthalmol Vis Sci 55, 3651–3659. - PubMed
-
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K, 1996. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271, 20246–20249. - PubMed
-
- Ambekar R, Toussaint KC Jr., Wagoner Johnson A, 2011. The effect of keratoconus on the structural, mechanical, and optical properties of the cornea. J Mech Behav Biomed Mater 4, 223–236. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

