Myosin light chain kinase is a potential target for hypopharyngeal cancer treatment
- PMID: 32920510
- PMCID: PMC8122670
- DOI: 10.1016/j.biopha.2020.110665
Myosin light chain kinase is a potential target for hypopharyngeal cancer treatment
Abstract
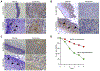
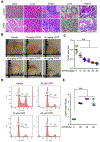
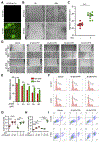
Hypopharyngeal cancer is squamous cell carcinoma (SCC) with the worst prognosis among the head and neck cancers. Overall, the 5-year survival rate remains poor although diagnostic imaging, radiation, chemotherapy, and surgical techniques have been improved. The mortality of patients with hypopharyngeal cancer is partly due to an increased likelihood of developing a second primary malignancy and metastasis. In this study, we found that MLCK expression, compared to healthy tissue, was up-regulated in hypopharyngeal tumor tissue. Of particular interest, a low 5-year survival rate was positively correlated with MLCK expression. We hypothesized that MLCK might be a target for hypopharyngeal cancer prognosis and treatment. In order to explore the function of MLCK in the development of cancer, we knockdown MLCK in hypopharyngeal cancer FaDu cells. The results showed that MLCK knockdown reduced the migration and invasion of FaDu cells. 4-amino-2-trifluoromethyl-phenyl retinate (ATPR) is the derivative of all-trans retinoic acid (ATRA), which was able to reduce both MLCK expression and activity in FaDu cells. ATPR induced FaDu cells apoptosis in a dose-dependent manner and also inhibited cell growth both in vivo and in vitro. Further experiments showed that overexpression of MLCK reduced ATPR induced-migration inhibition while increase of ATPR induced apoptosis, which suggested that MLCK was involved in ATPR's anti-cancer function. In conclusion, MLCK is a novel prognostic marker and therapeutic target for hypopharyngeal cancer. By targeting MLCK, ATPR exhibits its potential application in the treatment of this type of cancer.
Keywords: ATPR; ATRA; Hypopharyngeal cancer; MLCK.
Copyright © 2020 The Authors. Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors report no declarations of interest.
Figures



Similar articles
-
A novel all-trans retinoid acid derivative N-(3-trifluoromethyl- phenyl)- retinamide inhibits lung adenocarcinoma A549 cell migration through down-regulating expression of myosin light chain kinase.Asian Pac J Cancer Prev. 2014;15(18):7687-92. doi: 10.7314/apjcp.2014.15.18.7687. Asian Pac J Cancer Prev. 2014. PMID: 25292047
-
4-Amino-2-trifluoromethyl-phenyl retinate inhibits the migration of BGC-823 human gastric cancer cells by downregulating the phosphorylation level of MLC II.Oncol Rep. 2014 Oct;32(4):1473-80. doi: 10.3892/or.2014.3343. Epub 2014 Jul 18. Oncol Rep. 2014. PMID: 25051015
-
A novel all-trans retinoid acid derivatives inhibits the migration of breast cancer cell lines MDA-MB-231 via myosin light chain kinase involving p38-MAPK pathway.Biomed Pharmacother. 2013 Jun;67(5):357-62. doi: 10.1016/j.biopha.2013.03.016. Epub 2013 Apr 2. Biomed Pharmacother. 2013. PMID: 23602051
-
New insights into 4-amino-2-tri-fluoromethyl-phenyl ester inhibition of cell growth and migration in the A549 lung adenocarcinoma cell line.Asian Pac J Cancer Prev. 2013;14(12):7265-70. doi: 10.7314/apjcp.2013.14.12.7265. Asian Pac J Cancer Prev. 2013. PMID: 24460286
-
Long non-coding RNA PVT1 regulates TGF-β and promotes the proliferation, migration and invasion of hypopharyngeal carcinoma FaDu cells.World J Surg Oncol. 2024 Sep 20;22(1):254. doi: 10.1186/s12957-024-03536-w. World J Surg Oncol. 2024. PMID: 39300515 Free PMC article.
Cited by
-
Tight junctions: from molecules to gastrointestinal diseases.Tissue Barriers. 2023 Apr 3;11(2):2077620. doi: 10.1080/21688370.2022.2077620. Epub 2022 May 27. Tissue Barriers. 2023. PMID: 35621376 Free PMC article.
-
Critical role for the lung endothelial nonmuscle myosin light-chain kinase isoform in the severity of inflammatory murine lung injury.Pulm Circ. 2022 Apr 7;12(2):e12061. doi: 10.1002/pul2.12061. eCollection 2022 Apr. Pulm Circ. 2022. PMID: 35514774 Free PMC article.
-
A novel tumor suppressor role of myosin light chain kinase splice variants through downregulation of the TEAD4/CD44 axis.Carcinogenesis. 2021 Jul 16;42(7):961-974. doi: 10.1093/carcin/bgab038. Carcinogenesis. 2021. PMID: 34000008 Free PMC article.
-
Effect of forkhead box protein P2-mediated activation of myosin light-chain kinase on the invasion and migration of endometrial cancer cells.Cytojournal. 2025 May 14;22:54. doi: 10.25259/Cytojournal_31_2025. eCollection 2025. Cytojournal. 2025. PMID: 40539122 Free PMC article.
-
Kinase activities in pancreatic ductal adenocarcinoma with prognostic and therapeutic avenues.Mol Oncol. 2024 Aug;18(8):2020-2041. doi: 10.1002/1878-0261.13625. Epub 2024 Apr 22. Mol Oncol. 2024. PMID: 38650175 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

