Persistent Rheb-induced mTORC1 activation in spinal cord neurons induces hypersensitivity in neuropathic pain
- PMID: 32920594
- PMCID: PMC7487067
- DOI: 10.1038/s41419-020-02966-0
Persistent Rheb-induced mTORC1 activation in spinal cord neurons induces hypersensitivity in neuropathic pain
Abstract
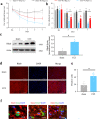
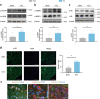
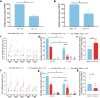
The small GTPase Ras homolog enriched in the brain (Rheb) can activate mammalian target of rapamycin (mTOR) and regulate the growth and cell cycle progression. We investigated the role of Rheb-mediated mTORC1 signaling in neuropathic pain. A chronic constriction injury (CCI) model was dopted. CCI induced obvious spinal Rheb expression and phosphorylation of mTOR, S6, and 4-E-BP1. Blocking mTORC1 signal with rapamycin alleviated the neuropathic pain and restored morphine efficacy in CCI model. Immunofluoresence showed a neuronal co-localization of CCI-induced Rheb and pS6. Rheb knockin mouse showed a similar behavioral phenotype as CCI. In spinal slice recording, CCI increased the firing frequency of neurons expressing HCN channels; inhibition of mTORC1 with rapamycin could reverse the increased spinal neuronal activity in neuropathic pain. Spinal Rheb is induced in neuropathic pain, which in turn active the mTORC1 signaling in CCI. Spinal Rheb-mTOR signal plays an important role in regulation of spinal sensitization in neuropathic pain, and targeting mTOR may give a new strategy for pain management.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Small G-Protein Rheb Gates Mammalian Target of Rapamycin Signaling to Regulate Morphine Tolerance in Mice.Anesthesiology. 2024 Apr 1;140(4):786-802. doi: 10.1097/ALN.0000000000004885. Anesthesiology. 2024. PMID: 38147625
-
Mammalian target of rapamycin in spinal cord neurons mediates hypersensitivity induced by peripheral inflammation.Neuroscience. 2010 Sep 1;169(3):1392-402. doi: 10.1016/j.neuroscience.2010.05.067. Epub 2010 Jun 9. Neuroscience. 2010. PMID: 20538043 Free PMC article.
-
ATF6 Regulates Cardiac Hypertrophy by Transcriptional Induction of the mTORC1 Activator, Rheb.Circ Res. 2019 Jan 4;124(1):79-93. doi: 10.1161/CIRCRESAHA.118.313854. Circ Res. 2019. PMID: 30582446 Free PMC article.
-
Amino acid regulation of TOR complex 1.Am J Physiol Endocrinol Metab. 2009 Apr;296(4):E592-602. doi: 10.1152/ajpendo.90645.2008. Epub 2008 Sep 2. Am J Physiol Endocrinol Metab. 2009. PMID: 18765678 Free PMC article. Review.
-
Coordination of Rheb lysosomal membrane interactions with mTORC1 activation.F1000Res. 2020 May 27;9:F1000 Faculty Rev-450. doi: 10.12688/f1000research.22367.1. eCollection 2020. F1000Res. 2020. PMID: 32518628 Free PMC article. Review.
Cited by
-
The mTOR inhibitor rapamycin suppresses trigeminal neuropathic pain and p-MKK4/p-p38 mitogen-activated protein kinase-mediated microglial activation in the trigeminal nucleus caudalis of mice with infraorbital nerve injury.Front Mol Neurosci. 2023 Apr 14;16:1172366. doi: 10.3389/fnmol.2023.1172366. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37122619 Free PMC article.
-
Epigenetic combined with transcriptomic analysis of the m6A methylome after spared nerve injury-induced neuropathic pain in mice.Neural Regen Res. 2023 Nov;18(11):2545-2552. doi: 10.4103/1673-5374.371374. Neural Regen Res. 2023. PMID: 37282488 Free PMC article.
-
IGF/mTORC1/S6 Signaling Is Potentiated and Prolonged by Acute Loading of Subtoxicological Manganese Ion.Biomolecules. 2023 Aug 8;13(8):1229. doi: 10.3390/biom13081229. Biomolecules. 2023. PMID: 37627294 Free PMC article.
-
Proteomic Analysis of the Spinal Dorsal Horn in Mice with Neuropathic Pain After Exercise.J Pain Res. 2023 Mar 18;16:973-984. doi: 10.2147/JPR.S403374. eCollection 2023. J Pain Res. 2023. PMID: 36968761 Free PMC article.
-
Increased miR-155 in Microglial Exosomes Following Heat Stress Accelerates Neuronal Autophagy via Their Transfer Into Neurons.Front Cell Neurosci. 2022 May 11;16:865568. doi: 10.3389/fncel.2022.865568. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35634460 Free PMC article.
References
-
- Groenewoud MJ, Zwartkruis FJ. Rheb and Rags come together at the lysosome to activate mTORC1. Biochem. Soc. Trans. 2013;41:951–955. - PubMed
-
- Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. - PubMed
-
- Jaggi AS, Singh N. Role of different brain areas in peripheral nerve injury-induced neuropathic pain. Brain Res. 2011;1381:187–201. - PubMed
-
- Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–819. - PubMed
-
- May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

