The role of the gut microbiome in graft fibrosis after pediatric liver transplantation
- PMID: 32920649
- PMCID: PMC8052232
- DOI: 10.1007/s00439-020-02221-8
The role of the gut microbiome in graft fibrosis after pediatric liver transplantation
Abstract
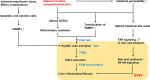
Liver transplantation (LT) is a life-saving option for children with end-stage liver disease. However, about 50% of patients develop graft fibrosis in 1 year after LT, with normal liver function. Graft fibrosis may progress to cirrhosis, resulting in graft dysfunction and ultimately the need for re-transplantation. Previous studies have identified various risk factors for the post-LT fibrogenesis, however, to date, neither of the factors seems to fully explain the cause of graft fibrosis. Recently, evidence has accumulated on the important role of the gut microbiome in outcomes after solid organ transplantation. As an altered microbiome is present in pediatric patients with end-stage liver diseases, we hypothesize that the persisting alterations in microbial composition or function contribute to the development of graft fibrosis, for example by bacteria translocation due to increased intestinal permeability, imbalanced bile acids metabolism, and/or decreased production of short-chain fatty acids (SCFAs). Subsequently, an immune response can be activated in the graft, together with the stimulation of fibrogenesis. Here we review current knowledge about the potential mechanisms by which alterations in microbial composition or function may lead to graft fibrosis in pediatric LT and we provide prospective views on the efficacy of gut microbiome manipulation as a therapeutic target to alleviate the graft fibrosis and to improve long-term survival after LT.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Alisi A, Bedogni G, Baviera G, Giorgio V, Porro E, Paris C, Giammaria P, Reali L, Anania F, Nobili V. Randomised clinical trial: the beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014;39:1276–1285. doi: 10.1111/apt.12758. - DOI - PMC - PubMed
-
- Annavajhala MK, Gomez-Simmonds A, Macesic N, Sullivan SB, Kress A, Khan SD, Giddins MJ, Stump S, Kim GI, Narain R, Verna EC, Uhlemann AC. Colonizing multidrug-resistant bacteria and the longitudinal evolution of the intestinal microbiome after liver transplantation. Nat Commun. 2019;10:4715. doi: 10.1038/s41467-019-12633-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

