Endothelial JAK2V617F mutation leads to thrombosis, vasculopathy, and cardiomyopathy in a murine model of myeloproliferative neoplasm
- PMID: 32920974
- PMCID: PMC7756295
- DOI: 10.1111/jth.15095
Endothelial JAK2V617F mutation leads to thrombosis, vasculopathy, and cardiomyopathy in a murine model of myeloproliferative neoplasm
Abstract
Objective: Cardiovascular complications are the leading cause of morbidity and mortality in patients with myeloproliferative neoplasms (MPNs). The acquired kinase mutation JAK2V617F plays a central role in these disorders. Mechanisms responsible for cardiovascular dysfunction in MPNs are not fully understood, limiting the effectiveness of current treatment. Vascular endothelial cells (ECs) carrying the JAK2V617F mutation can be detected in patients with MPNs. The goal of this study was to test the hypothesis that the JAK2V617F mutation alters endothelial function to promote cardiovascular complications in patients with MPNs.
Approach and results: We employed murine models of MPN in which the JAK2V617F mutation is expressed in specific cell lineages. When JAK2V617F is expressed in both blood cells and vascular ECs, the mice developed MPN and spontaneous, age-related dilated cardiomyopathy with an increased risk of sudden death as well as a prothrombotic and vasculopathy phenotype on histology evaluation. In contrast, despite having significantly higher leukocyte and platelet counts than controls, mice with JAK2V617F-mutant blood cells alone did not demonstrate any cardiac dysfunction, suggesting that JAK2V617F-mutant ECs are required for this cardiovascular disease phenotype. Furthermore, we demonstrated that the JAK2V617F mutation promotes a pro-adhesive, pro-inflammatory, and vasculopathy EC phenotype, and mutant ECs respond to flow shear differently than wild-type ECs.
Conclusions: These findings suggest that the JAK2V617F mutation can alter vascular endothelial function to promote cardiovascular complications in MPNs. Therefore, targeting the MPN vasculature represents a promising new therapeutic strategy for patients with MPNs.
Keywords: cardiomyopathy; endothelial cells; myeloproliferative disorders; thrombosis; vascular diseases.
© 2020 The Authors. Journal of Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis.
Conflict of interest statement
The authors have declared that no conflicts of interest exists.
Figures

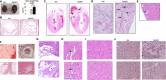



References
-
- Landolfi R, Di Gennaro L, Falanga A. Thrombosis in myeloproliferative disorders: pathogenetic facts and speculation. Leukemia. 2008;22:2020‐2028. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

