The Brain Chart of Aging: Machine-learning analytics reveals links between brain aging, white matter disease, amyloid burden, and cognition in the iSTAGING consortium of 10,216 harmonized MR scans
- PMID: 32920988
- PMCID: PMC7923395
- DOI: 10.1002/alz.12178
The Brain Chart of Aging: Machine-learning analytics reveals links between brain aging, white matter disease, amyloid burden, and cognition in the iSTAGING consortium of 10,216 harmonized MR scans
Abstract
Introduction: Relationships between brain atrophy patterns of typical aging and Alzheimer's disease (AD), white matter disease, cognition, and AD neuropathology were investigated via machine learning in a large harmonized magnetic resonance imaging database (11 studies; 10,216 subjects).
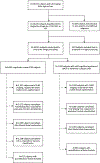
Methods: Three brain signatures were calculated: Brain-age, AD-like neurodegeneration, and white matter hyperintensities (WMHs). Brain Charts measured and displayed the relationships of these signatures to cognition and molecular biomarkers of AD.
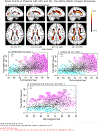
Results: WMHs were associated with advanced brain aging, AD-like atrophy, poorer cognition, and AD neuropathology in mild cognitive impairment (MCI)/AD and cognitively normal (CN) subjects. High WMH volume was associated with brain aging and cognitive decline occurring in an ≈10-year period in CN subjects. WMHs were associated with doubling the likelihood of amyloid beta (Aβ) positivity after age 65. Brain aging, AD-like atrophy, and WMHs were better predictors of cognition than chronological age in MCI/AD.
Discussion: A Brain Chart quantifying brain-aging trajectories was established, enabling the systematic evaluation of individuals' brain-aging patterns relative to this large consortium.
Keywords: Alzheimer's disease pathology; Dementia; MRI; Machine Learning; Neuroimaging; PET; beta-amyloid; brain aging; brain signatures; cognitive testing; harmonized neuroimaging cohorts; preclinical Alzheimer's disease; small vessel ischemic disease; tau.
© 2020 the Alzheimer's Association.
Conflict of interest statement
Conflict of interest statements
None
Figures




References
-
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal Magnetic Resonance Imaging Studies of Older Adults: A Shrinking Brain. J Neurosci. 2003;23(8):3295–3301. http://www.jneurosci.org/content/23/8/3295.abstract - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EB022573/EB/NIBIB NIH HHS/United States
- U01 AG068057/AG/NIA NIH HHS/United States
- U19 AG033655/AG/NIA NIH HHS/United States
- P30 AG049638/AG/NIA NIH HHS/United States
- RF1 AG059869/AG/NIA NIH HHS/United States
- RF1 AG054409/AG/NIA NIH HHS/United States
- HHSN271201600059C/HL/NHLBI NIH HHS/United States
- S10 OD023495/OD/NIH HHS/United States
- P30 AG066507/AG/NIA NIH HHS/United States
- HHSN271201600059C/DA/NIDA NIH HHS/United States
- P30 AG062715/AG/NIA NIH HHS/United States
- R01 AG063887/AG/NIA NIH HHS/United States

