Phosphorylated Alpha-Synuclein Within Cutaneous Autonomic Nerves of Patients With Parkinson's Disease: The Implications of Sample Thickness on Results
- PMID: 32921251
- PMCID: PMC7534099
- DOI: 10.1369/0022155420960250
Phosphorylated Alpha-Synuclein Within Cutaneous Autonomic Nerves of Patients With Parkinson's Disease: The Implications of Sample Thickness on Results
Abstract
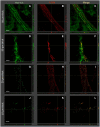
The detection of cutaneous phosphorylated alpha-synuclein (P-syn) in patients with Parkinson's disease (PD) has ranged from 30% to 100% across different studies. We hypothesize that part of the variability in P-syn detection is due to methodological differences using sections of different tissue thickness. Three skin biopsies were obtained from 29 individuals with PD and 21 controls. Tissues were cut into 10-, 20-, and 50-µm-thick sections and double-stained with protein gene product (PGP) 9.5 and P-syn. We quantified the deposition of P-syn with and without PGP 9.5 in sweat glands, pilomotor muscle, and blood vessels using confocal digital images of autonomic structures. Overall, the P-syn-positive rates with PGP 9.5 colocalization in subjects with PD were 100% using 50 µm sections, 90% using 20 µm sections, and 73% using 10 µm sections with 100% specificity. (No P-syn was detected within control subjects.) Without PGP 9.5, colocalization of the P-syn-positive rates was 100% for all samples, but specificity dropped below 70%. In this study, double-immunostained 50 µm skin biopsy tissue sections are superior to 20 and 10 µm tissue sections at detecting P-syn in subjects with PD. The increased sensitivity is likely secondary to a combination of greater volume of tissue analyzed and improved visualization of nerve fiber architecture.
Keywords: Parkinson’s disease; autonomic nerve system; phosphorylated alpha-synuclein-s129; skin biopsy.
Figures

References
-
- Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, Eschbacher J, White CL, III, Akiyama H, Caviness J, Shill HA, Connor DJ, Sabbagh MN, Walker DG. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117(6):613–634. doi:10.1007/s00401-009-0538-8 - DOI - PMC - PubMed
-
- Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White CL, III, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, Walker DG. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119(6):689–702. doi:10.1007/s00401-010-0664-3 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

