Single- and Multifraction Stereotactic Radiosurgery Dose/Volume Tolerances of the Brain
- PMID: 32921513
- PMCID: PMC9387178
- DOI: 10.1016/j.ijrobp.2020.08.013
Single- and Multifraction Stereotactic Radiosurgery Dose/Volume Tolerances of the Brain
Abstract
Purpose: As part of the American Association of Physicists in Medicine Working Group on Stereotactic Body Radiotherapy investigating normal tissue complication probability (NTCP) after hypofractionated radiation therapy, data from published reports (PubMed indexed 1995-2018) were pooled to identify dosimetric and clinical predictors of radiation-induced brain toxicity after single-fraction stereotactic radiosurgery (SRS) or fractionated stereotactic radiosurgery (fSRS).
Methods and materials: Eligible studies provided NTCPs for the endpoints of radionecrosis, edema, or symptoms after cranial SRS/fSRS and quantitative dose-volume metrics. Studies of patients with only glioma, meningioma, vestibular schwannoma, or brainstem targets were excluded. The data summary and analyses focused on arteriovenous malformations (AVM) and brain metastases.
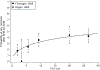
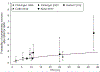
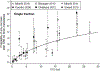
Results: Data from 51 reports are summarized. There was wide variability in reported rates of radionecrosis. Available data for SRS/fSRS for brain metastases were more amenable to NTCP modeling than AVM data. In the setting of brain metastases, SRS/fSRS-associated radionecrosis can be difficult to differentiate from tumor progression. For single-fraction SRS to brain metastases, tissue volumes (including target volumes) receiving 12 Gy (V12) of 5 cm3, 10 cm3, or >15 cm3 were associated with risks of symptomatic radionecrosis of approximately 10%, 15%, and 20%, respectively. SRS for AVM was associated with modestly lower rates of symptomatic radionecrosis for equivalent V12. For brain metastases, brain plus target volume V20 (3-fractions) or V24 (5-fractions) <20 cm3 was associated with <10% risk of any necrosis or edema, and <4% risk of radionecrosis requiring resection.
Conclusions: The risk of radionecrosis after SRS and fSRS can be modeled as a function of dose and volume treated. The use of fSRS appears to reduce risks of radionecrosis for larger treatment volumes relative to SRS. More standardized dosimetric and toxicity reporting is needed to facilitate future pooled analyses that can refine predictive models of brain toxicity risks.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest:
Figures





References
-
- Fabiano AJ, Prasad D Qiu J. Adverse radiation effect in the brain during cancer radiotherapy. J Radiat Cancer Res 2017;8:135–140.
-
- Schultheiss TE, Kun LE, Ang KK, et al. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys 1995;31:1093–1112. - PubMed
-
- Chao ST, Ahluwalia MS, Barnett GH, et al. Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol Biol Phys 2013;87:449–457. - PubMed
-
- Mehta S, Shah A Jung H. Diagnosis and treatment options for sequelae following radiation treatment of brain tumors. Clinical neurology and neurosurgery 2017;163:1–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

