Emerging roles of the MAGE protein family in stress response pathways
- PMID: 32921631
- PMCID: PMC7681028
- DOI: 10.1074/jbc.REV120.008029
Emerging roles of the MAGE protein family in stress response pathways
Abstract
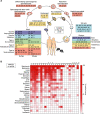
The melanoma antigen (MAGE) proteins all contain a MAGE homology domain. MAGE genes are conserved in all eukaryotes and have expanded from a single gene in lower eukaryotes to ∼40 genes in humans and mice. Whereas some MAGEs are ubiquitously expressed in tissues, others are expressed in only germ cells with aberrant reactivation in multiple cancers. Much of the initial research on MAGEs focused on exploiting their antigenicity and restricted expression pattern to target them with cancer immunotherapy. Beyond their potential clinical application and role in tumorigenesis, recent studies have shown that MAGE proteins regulate diverse cellular and developmental pathways, implicating them in many diseases besides cancer, including lung, renal, and neurodevelopmental disorders. At the molecular level, many MAGEs bind to E3 RING ubiquitin ligases and, thus, regulate their substrate specificity, ligase activity, and subcellular localization. On a broader scale, the MAGE genes likely expanded in eutherian mammals to protect the germline from environmental stress and aid in stress adaptation, and this stress tolerance may explain why many cancers aberrantly express MAGEs Here, we present an updated, comprehensive review on the MAGE family that highlights general characteristics, emphasizes recent comparative studies in mice, and describes the diverse functions exerted by individual MAGEs.
Keywords: AMP-activated kinase (AMPK); DNA damage response; E3 ligase; E3 ubiquitin ligase; Fe-S cluster; MAGE; Prader-Willi syndrome; Schaaf-Yang syndrome; alternative polyadenylation; apoptosis; cancer; cancer-testis antigen; cell metabolism; melanoma antigen; metabolism; p53; spermatogenesis; stress adaptation; stress granule; stress response; ubiquitin; ubiquitination.
© 2020 Florke Gee et al.
Conflict of interest statement
Conflict of interest—P. R. P. is a paid consultant of Levo Therapeutics, Inc. and Amgen, Inc.
Figures




References
-
- Chomez P., De Backer O., Bertrand M., De Plaen E., Boon T., and Lucas S. (2001) An overview of the mage gene family with the identification of all human members of the family. Cancer Res. 61, 5544–5551 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

