SARS-CoV-2 recurrent RNA positivity after recovering from coronavirus disease 2019 (COVID-19): a meta-analysis
- PMID: 32921710
- PMCID: PMC7717013
- DOI: 10.23750/abm.v91i3.10303
SARS-CoV-2 recurrent RNA positivity after recovering from coronavirus disease 2019 (COVID-19): a meta-analysis
Abstract
Background and aim: Isolation of subjects with active severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is a pivotal preventive measure in the ongoing coronavirus disease 2019 (COVID-19) pandemic. A growing number of studies reported cases of recurrent SARS-CoV-2 RNA positivity following disease recovery, which were identified with a critical literature search and then meta-analyzed in this article.
Materials and methods: A digital search was performed in Medline and Web of Science, using the keywords "coronavirus disease 2019" OR "COVID-19" OR "severe acute respiratory disease 2" OR "SARS-CoV-2" AND "recurrence" OR "repositivization" OR "retesting", without date or language restrictions. Recovery was defined as resolution of symptoms, with at least two consecutive negative molecular tests.
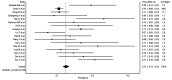
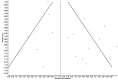
Results: A total number of 17 studies, with 5,182 COVID-19 patients, were included. SARS-CoV-2 recurrent RNA positivity in recovered COVID-19 patients ranged between 7-23% across the studies, with follow-up testing between 1-60 days. The estimated cumulative rate of SARS-CoV-2 recurrent RNA positivity was 12% (95% confidence interval, 12-13%; I2, 74%).
Conclusions: Repeated molecular testing on respiratory tracts specimens at 1 and 2 months after recovery from COVID-19 is strongly advisable for early identification, isolation and clinical management of subjects with SARS-CoV-2 recurrent RNA positivity.
Conflict of interest statement
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
Figures
Comment in
-
Retest positive for SARS-CoV-2 RNA in pregnant women recovered from COVID-19.Ultrasound Obstet Gynecol. 2020 Dec;56(6):948-949. doi: 10.1002/uog.23144. Ultrasound Obstet Gynecol. 2020. PMID: 33034931 Free PMC article. No abstract available.
Similar articles
-
COVID-19: a conundrum to decipher.Eur Rev Med Pharmacol Sci. 2020 May;24(10):5830-5841. doi: 10.26355/eurrev_202005_21378. Eur Rev Med Pharmacol Sci. 2020. PMID: 32495923
-
Viral RNA level, serum antibody responses, and transmission risk in recovered COVID-19 patients with recurrent positive SARS-CoV-2 RNA test results: a population-based observational cohort study.Emerg Microbes Infect. 2020 Dec;9(1):2368-2378. doi: 10.1080/22221751.2020.1837018. Emerg Microbes Infect. 2020. PMID: 33151135 Free PMC article.
-
Duration of Respiratory and Gastrointestinal Viral Shedding in Children With SARS-CoV-2: A Systematic Review and Synthesis of Data.Pediatr Infect Dis J. 2020 Sep;39(9):e249-e256. doi: 10.1097/INF.0000000000002814. Pediatr Infect Dis J. 2020. PMID: 32618932
-
A case presentation for positive SARS-CoV-2 RNA recurrence in a patient with a history of type 2 diabetes that had recovered from severe COVID-19.Diabetes Res Clin Pract. 2020 Aug;166:108300. doi: 10.1016/j.diabres.2020.108300. Epub 2020 Jul 12. Diabetes Res Clin Pract. 2020. PMID: 32663490 Free PMC article.
-
Prevalence of Gastrointestinal Symptoms and Fecal Viral Shedding in Patients With Coronavirus Disease 2019: A Systematic Review and Meta-analysis.JAMA Netw Open. 2020 Jun 1;3(6):e2011335. doi: 10.1001/jamanetworkopen.2020.11335. JAMA Netw Open. 2020. PMID: 32525549 Free PMC article.
Cited by
-
A systematic review on the recurrence of SARS-CoV-2 virus: frequency, risk factors, and possible explanations.Infect Dis (Lond). 2021 May;53(5):315-324. doi: 10.1080/23744235.2020.1871066. Epub 2021 Jan 28. Infect Dis (Lond). 2021. PMID: 33508989 Free PMC article.
-
Elucidating reasons of COVID-19 re-infection and its management strategies.Diabetes Metab Syndr. 2021 May-Jun;15(3):1001-1006. doi: 10.1016/j.dsx.2021.05.008. Epub 2021 May 7. Diabetes Metab Syndr. 2021. PMID: 33989898 Free PMC article. Review.
-
A Case Series Describing the Recurrence of COVID-19 in Patients Who Recovered from Initial Illness in Bangladesh.Trop Med Infect Dis. 2021 Mar 31;6(2):41. doi: 10.3390/tropicalmed6020041. Trop Med Infect Dis. 2021. PMID: 33807247 Free PMC article.
-
Reinfection rates among patients previously infected by SARS-CoV-2: systematic review and meta-analysis.Chin Med J (Engl). 2022 Jan 20;135(2):145-152. doi: 10.1097/CM9.0000000000001892. Chin Med J (Engl). 2022. PMID: 34908003 Free PMC article.
-
Retest positive for SARS-CoV-2 RNA in pregnant women recovered from COVID-19.Ultrasound Obstet Gynecol. 2020 Dec;56(6):948-949. doi: 10.1002/uog.23144. Ultrasound Obstet Gynecol. 2020. PMID: 33034931 Free PMC article. No abstract available.
References
-
- Khullar D, Bond AM, Schpero WL. COVID-19 and the Financial Health of US Hospitals. JAMA. 2020 May 4 doi: 10.1001/jama.2020.6269. Epub ahead of print. - PubMed
-
- Lippi G, Adeli K, Ferrari M, et al. Biosafety measures for preventing infection from COVID-19 in clinical laboratories: IFCC Taskforce Recommendations. Clin Chem Lab Med. 2020;58:1053–1062. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous