RT-qPCR assays based on saliva rather than on nasopharyngeal swabs are possible but should be interpreted with caution: results from a systematic review and meta-analysis
- PMID: 32921721
- PMCID: PMC7717018
- DOI: 10.23750/abm.v91i3.10020
RT-qPCR assays based on saliva rather than on nasopharyngeal swabs are possible but should be interpreted with caution: results from a systematic review and meta-analysis
Abstract
Background and aim of the work: The ongoing pandemic has elicited an increasing interest regarding the SARS-CoV-2 viral RNA detection in saliva specimens rather than through nasopharyngeal swabs. Our aim was to conduct a meta-analysis on the sensitivity and specificity of SARS-CoV-2 viral RNA detection through RT-qPCR based on salivary specimens compared to conventional nasopharyngeal swabs.
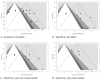
Methods: We reported our meta-analysis according to the PRISMA statement. We searched Pubmed, Embase, and pre-print archive medRxiv.og for eligible studies published up to June 1st, 2020. Raw data included true/false positive and negative tests, and the total number of tests. Sensitivity and specificity data were calculated for every study, and then pooled in a random-effects model. Heterogeneity was assessed using the I2 measure. Reporting bias was assessed by means of funnel plots and regression analysis.
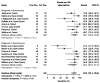
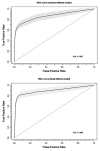
Results: The systematic review eventually retrieved 14 studies including a total of 15 estimates, the were included in quantitative synthesis. We found a pooled specificity of 97.7% (95%CI 93.8-99.2) and a pooled sensitivity of 83.4% (95%CI 73.1-90.4), with an overall agreement assessed by means of Cohen's kappa equals to 0.750, 95%CI 0.62-0.88 (i.e. moderate agreement), with high heterogeneity and risk of reporting bias.
Conclusions: In conclusion, diagnostic tests based on salivary specimens are somewhat reliable, but relatively few studies have been carried out. Moreover, such studies are characterized by low numbers and low sample power. Therefore, the of salivary samples is currently questionable for clinical purposes and cannot substitute other more conventional RT-qPCR based on nasopharyngeal swabs.
Conflict of interest statement
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article. The facts, conclusions, and opinions stated in the article represent the authors’ research, conclusions, and opinions and are believed to be substantiated, accurate, valid, and reliable. However, as this article includes the results of personal researches of the Authors, presenting correspondent, personal conclusions and opinions, parent employers are not forced in any way to endorse or share its content and its potential implications.
Figures




References
-
- European Centre for Diseases Prevention and Control (ECDC) Stockholm: 2020. An overview of the rapid test situation for COVID-19 diagnosis in the EU / EEA. available from: https://www.ecdc.europa.eu/en/publications-data/overview-rapid-test-situ.... (accessed June 9, 2020)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

