Tumor Microenvironmental Responsive Liposomes Simultaneously Encapsulating Biological and Chemotherapeutic Drugs for Enhancing Antitumor Efficacy of NSCLC
- PMID: 32922011
- PMCID: PMC7457883
- DOI: 10.2147/IJN.S258906
Tumor Microenvironmental Responsive Liposomes Simultaneously Encapsulating Biological and Chemotherapeutic Drugs for Enhancing Antitumor Efficacy of NSCLC
Erratum in
-
Erratum: Tumor Microenvironmental Responsive Liposomes Simultaneously Encapsulating Biological and Chemotherapeutic Drugs for Enhancing Antitumor Efficacy of NSCLC [Corrigendum].Int J Nanomedicine. 2021 Dec 14;16:8067-8068. doi: 10.2147/IJN.S353086. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34938073 Free PMC article.
Abstract
Background: Non-small cell lung cancer (NSCLC) is one of the most lethal types of cancer with highly infiltrating. Chemotherapy is far from satisfactory, vasculogenic mimicry (VM) and angiogenesis results in invasion, migration and relapse.
Purpose: The objective of this study was to construct a novel CPP (mmp) modified vinorelbine and dioscin liposomes by two new functional materials, DSPE-PEG2000-MAL and CPP-PVGLIG-PEG5000, to destroy VM channels, angiogenesis, EMT and inhibit invasion and migration.
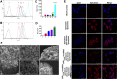
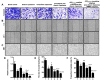
Methods and results: The targeting liposomes could be enriched in tumor sites through passive targeting, and the positively charged CPP was exposed and enhanced active targeting via electrostatic adsorption after being hydrolyzed by MMP2 enzymes overexpressed in the tumor microenvironment. We found that CPP (mmp) modified vinorelbine and dioscin liposomes with the ideal physicochemical properties and exhibited enhanced cellular uptake. In vitro and in vivo results showed that CPP (mmp) modified vinorelbine and dioscin liposomes could inhibit migration and invasion of A549 cells, destroy VM channels formation and angiogenesis, and block the EMT process. Pharmacodynamic studies showed that the targeting liposomes had obvious accumulations in tumor sites and magnificent antitumor efficiency.
Conclusion: CPP (mmp) modified vinorelbine plus dioscin liposomes could provide a new strategy for NSCLC.
Keywords: MMP2 enzymes; dioscin; multi-functional liposomes; non-small cell lung cancer; tumor microenvironment; vinorelbine.
© 2020 Kong et al.
Conflict of interest statement
All authors declare that there are no conflicts of interest.
Figures






References
-
- Chan AWH, Tong JHM, Kwan JSH, et al. Assessment of programmed cell death ligand-1 expression by 4 diagnostic assays and its clinicopathological correlation in a large cohort of surgical resected non-small cell lung carcinoma. Mod Pathol. 2018;31:1381–1390. doi: 10.1038/s41379-018-0053-3 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

