Catalogue, distribution, taxonomic notes, and conservation of the Western Palearctic endemic hunchback beetles (Tenebrionidae, Misolampus)
- PMID: 32922132
- PMCID: PMC7458947
- DOI: 10.3897/zookeys.963.53500
Catalogue, distribution, taxonomic notes, and conservation of the Western Palearctic endemic hunchback beetles (Tenebrionidae, Misolampus)
Abstract
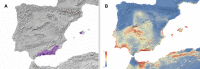
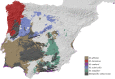
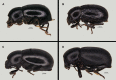
Hunchback darkling beetles of the Ibero-Maghrebian genus Misolampus Latreille, 1807 (Tenebrionidae, Stenochiinae) encompass six species: M. gibbulus (Herbst, 1799), M. goudotii Guérin-Méneville, 1834, M. lusitanicus Brême, 1842, M. ramburii Brême, 1842, M. scabricollis Graells, 1849, and M. subglaber Rosenhauer, 1856. Previously known distribution ranges of the species were delineated using many old records, the persistence of such populations being questionable under the current situation of global biodiversity loss. Additionally, the status of geographically isolated populations of the genus have been the subject of taxonomic controversy. An exhaustive bibliographical revision and field search was undertaken, and the Misolampus collection of the Museo Nacional de Ciencias Naturales (MNCN-CSIC) was revised. The aims are to (i) provide an updated geographic distribution range for the species of Misolampus; (ii) to determine the taxonomic status of controversial populations; (iii) to provide a catalogue for Misolampus; and (iv) to discuss the conservation status of these saproxylic beetles. As a result, a catalogue including synonymies and type localities, geographical records, diagnoses, and information on natural history for all species of Misolampus is presented. The results reveal that the distribution ranges of the species of Misolampus have not undergone a reduction in the last century, and indicate the presence of the genus in areas where it had never been recorded before. The morphological variability of M. goudotii drove the proposal of different taxa that are here formally synonymised as follows: M. goudotii Guérin-Méneville, 1834 = M. erichsoni Vauloger de Beaupré, 1900, syn. nov. = M. peyerimhoffi Antoine, 1926, syn. nov.
Keywords: Coleoptera; Stenochiinae; geographic range; morphological variability; new synonymies; population persistence; saproxylic; scientific collections.
Natalia Rosas-Ramos, Paloma Mas-Peinado, Diego Gil-Tapetado, Ernesto Recuero, José L. Ruiz, Mario García-París.
Figures














Similar articles
-
On the nomenclatural status of type genera in Coleoptera (Insecta).Zookeys. 2024 Jan 13;1194:1-981. doi: 10.3897/zookeys.1194.106440. eCollection 2024. Zookeys. 2024. PMID: 38523865 Free PMC article.
-
Review of genus-group names in the family Tenebrionidae (Insecta, Coleoptera).Zookeys. 2021 Jul 26;1050:1-633. doi: 10.3897/zookeys.1050.64217. eCollection 2021. Zookeys. 2021. PMID: 34385881 Free PMC article.
-
Hydroids (Cnidaria, Hydrozoa) from Mauritanian Coral Mounds.Zootaxa. 2020 Nov 16;4878(3):zootaxa.4878.3.2. doi: 10.11646/zootaxa.4878.3.2. Zootaxa. 2020. PMID: 33311142
-
A catalogue of the tribe Sepidiini Eschscholtz, 1829 (Tenebrionidae, Pimeliinae) of the world.Zookeys. 2019 May 13;844:1-121. doi: 10.3897/zookeys.844.34241. eCollection 2019. Zookeys. 2019. PMID: 31143077 Free PMC article.
-
A taxonomic review of the genus Euboeus Boieldieu, 1865 (Coleoptera: Tenebrionidae: Helopini) of the Caucasus, Iran and Turkmenistan.Zootaxa. 2022 Jul 1;5159(4):451-486. doi: 10.11646/zootaxa.5159.4.1. Zootaxa. 2022. PMID: 36095536 Review.
References
-
- Abellán P, Svenning JC. (2014) Refugia within refugia – patterns in endemism and genetic divergence are linked to Late Quaternary climate stability in the Iberian Peninsula. Biological Journal of the Linnean Society 113(1): 13–28. 10.1111/bij.12309 - DOI
-
- Alcaráz Ariza F, Peinado Lorca M. (1987) España semiárida: Murcia y Almería. In: Peinado Lorca M, Rivas-Martínez S. (Eds) La vegetación de España.Servicio de Publicaciones, Universidad de Alcalá de Henares, San Fernando de Henares (Madrid), 257–281.
-
- Antoine M. (1926) Notes d’entomologie marocaine. VI (1) Tenebrionides nouveaux et intéressants. Bulletin de la Société des Sciences Naturelles du Maroc 5: 248–259. https://bibdigital.rjb.csic.es/viewer/12177/?offset=#page=302&viewer=pic...
-
- Antoine M. (1949) Notes d’entomologie marocaine. 46 Materiaux pour l’étude des Helopinae du Maroc (Col. Tenebrionides). Bulletin de la Société des Sciences Naturelles du Maroc 27 [1947]: 123–162. https://bibdigital.rjb.csic.es/viewer/16061/?offset=#page=137&viewer=pic...
-
- Antoine M. (1954) Notes d’entomologie marocaine. LX: sur quelques captures intéressantes (Coléoptères Carabiques et Tenebrionidae). Bulletin de la Société des Sciences Naturelles et Physiques du Maroc 34: 199–209.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
