Stellate Ganglion Blockade repairs Intestinal Mucosal Barrier through suppression of Endoplasmic Reticulum Stress following Hemorrhagic Shock
- PMID: 32922175
- PMCID: PMC7484657
- DOI: 10.7150/ijms.47662
Stellate Ganglion Blockade repairs Intestinal Mucosal Barrier through suppression of Endoplasmic Reticulum Stress following Hemorrhagic Shock
Abstract
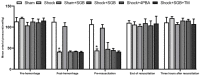
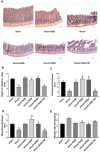
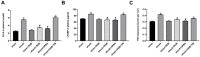
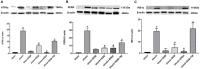
Background: Hemorrhagic shock-induced ischemia and hypoxia elicit endoplasmic reticulum stress (ERS) that leads to cell apoptosis, tissue structural damage and organ dysfunction and failure. Stellate ganglion blockade (SGB) has been demonstrated to improve intestinal barrier dysfunction induced by hemorrhagic shock. The present study sought to investigate whether the beneficial effect of SGB on the intestinal mucosal barrier function is via suppression of ERS. Materials and methods: A conscious rat model of hemorrhagic shock (40 ±2 mmHg for 1 hour, followed by resuscitation) was established. The parameters reflecting intestinal morphology and intestinal mucosal barrier function including wet-dry ratio (W/D), intestinal permeability, D-lactic acid (D-LA) and intestinal fatty acid binding protein (I-FABP) in plasma, and expressions of ATF6α, PERK, and IRE1α in intestinal tissues were then observed. Furthermore, the effects of either SGB or ERS inhibitor, 4-phenylbutyric acid (4-PBA), on these parameters in rats with hemorrhagic shock were assessed. The effect of ERS agonist tunicamycin (TM) on the rats subjected with both SGB and hemorrhagic shock was also determined. Results: Either SGB or administration of ERS inhibitor, 4-PBA, alleviated hemorrhagic shock-induced adverse effects such as intestinal mucosal barrier dysfunction and excessive autophagy, which were characterized by damaged intestinal tissue, enhanced intestinal permeability and D-LA and I-FABP levels in plasma, and increased expressions of ATF6α, PERK, IRE1α in intestinal tissue. In contrast, administration of ERS agonist, TM, suppressed the beneficial effects of SGB on intestinal tissue and function during hemorrhagic shock. Conclusion: The SGB repairs intestinal mucosal barrier through suppression of ERS following hemorrhagic shock.
Keywords: Endoplasmic reticulum stress; Hemorrhagic shock; Intestinal mucosal barrier; Stellate ganglion blockade.
© The author(s).
Conflict of interest statement
Competing Interests: No benefits in any form have been received or will be received from a commercial association related directly or indirectly to the subject of this article. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Figures
Similar articles
-
Blockade of Stellate Ganglion Remediates Hemorrhagic Shock-Induced Intestinal Barrier Dysfunction.J Surg Res. 2019 Dec;244:69-76. doi: 10.1016/j.jss.2019.06.007. Epub 2019 Jul 4. J Surg Res. 2019. PMID: 31279996
-
Stellate Ganglion Block Improves the Proliferation and Function of Splenic CD4 + T Cells Through Inhibition of Posthemorrhagic Shock Mesenteric Lymph-Mediated Autophagy.Inflammation. 2021 Dec;44(6):2543-2553. doi: 10.1007/s10753-021-01523-x. Epub 2021 Sep 17. Inflammation. 2021. PMID: 34533673
-
Stellate ganglion block ameliorates vascular calcification by inhibiting endoplasmic reticulum stress.Life Sci. 2018 Jan 15;193:1-8. doi: 10.1016/j.lfs.2017.12.002. Epub 2017 Dec 5. Life Sci. 2018. PMID: 29208463
-
[Induction mechanism of shock: applying the etiology in judgment of the cause of death in forensic practice].Nihon Hoigaku Zasshi. 2004 Sep;58(2):130-40. Nihon Hoigaku Zasshi. 2004. PMID: 15526767 Review. Japanese.
-
Induction mechanisms of autophagy and endoplasmic reticulum stress in intestinal ischemia-reperfusion injury, inflammatory bowel disease, and colorectal cancer.Biomed Pharmacother. 2024 Jan;170:115984. doi: 10.1016/j.biopha.2023.115984. Epub 2023 Dec 8. Biomed Pharmacother. 2024. PMID: 38070244 Review.
Cited by
-
Remifentanil Promotes PDIA3 Expression by Activating p38MAPK to Inhibit Intestinal Ischemia/Reperfusion-Induced Oxidative and Endoplasmic Reticulum Stress.Front Cell Dev Biol. 2022 Jan 26;10:818513. doi: 10.3389/fcell.2022.818513. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35155431 Free PMC article.
-
Autophagy Is Involved in Stellate Ganglion Block Reversing Posthemorrhagic Shock Mesenteric Lymph-Mediated Vascular Hyporeactivity.Front Physiol. 2021 Sep 21;12:728191. doi: 10.3389/fphys.2021.728191. eCollection 2021. Front Physiol. 2021. PMID: 34621184 Free PMC article.
-
Effects of ultrasound-guided stellate ganglion block on intrapulmonary shunt and oxygenation in patients with single-lung ventilation.Front Surg. 2024 Dec 23;11:1438146. doi: 10.3389/fsurg.2024.1438146. eCollection 2024. Front Surg. 2024. PMID: 39764215 Free PMC article.
-
miRNA-143 expression is associated with inflammation and time of exposure to amniotic fluid in experimental gastroschisis.Clinics (Sao Paulo). 2023 Nov 25;78:100311. doi: 10.1016/j.clinsp.2023.100311. eCollection 2023. Clinics (Sao Paulo). 2023. PMID: 38008037 Free PMC article.
-
Controlled Hemorrhage Sensitizes Angiotensin II-Elicited Hypertension through Activation of the Brain Renin-Angiotensin System Independently of Endoplasmic Reticulum Stress.Oxid Med Cell Longev. 2022 Jan 13;2022:6371048. doi: 10.1155/2022/6371048. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35069977 Free PMC article.
References
-
- Cannon JW. Hemorrhagic shock. N Engl J Med. 2018;378:370–9. - PubMed
-
- Leung CH, Caldarone CA, Wang F, Venkateswaran S, Ailenberg M, Vadasz B, Wen XY, Rotstein OD. Remote ischemic conditioning prevents lung and liver injury after hemorrhagic shock/Resuscitation: potential role of a humoral plasma factor. Ann Surg. 2015;261:1215–25. - PubMed
-
- Zhang J, Lin XR, Zhang YP, Zhang LM, Du HB, Jiang LN, Zhao ZG, Niu CY. Blockade of stellate ganglion remediates hemorrhagic shock-induced intestinal barrier dysfunction. J Surg Res. 2019;244:69–76. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

