Wheel-Running Behavior Is Negatively Impacted by Zinc Administration in a Novel Dual Transgenic Mouse Model of AD
- PMID: 32922260
- PMCID: PMC7456872
- DOI: 10.3389/fnins.2020.00854
Wheel-Running Behavior Is Negatively Impacted by Zinc Administration in a Novel Dual Transgenic Mouse Model of AD
Abstract
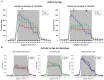
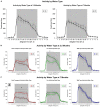
Alzheimer's disease (AD) is a neurocognitive disorder that impacts both the brain and behavior. Metal ions, including zinc (Zn), have been seen to play an important role in AD-related pathology. In this study, we show alterations in wheel-running behavior both early and late in disease progression in a novel dual Tg mouse model of AD. This mouse includes both amyloid and tau pathology through its cross with the J20 (hAPP) and P301L (Tau) parentage. Animals were given either lab water or water that had been supplemented with 10 ppm Zn. Wheel running was assessed through individually housing mice and measuring wheel-running activity in both the light and dark cycles. Dual Tg mice showed significantly less activity in the first part of the dark cycle than WT mice at both 3.5 and 7 months of age (p < 0.05). Dual Tg mice given Zn water showed less activity compared to dual Tg mice on lab water, tau mice on Zn water, or WT mice given either lab or Zn water (p < 0.05) at 7 months. Female mice in this study consistently showed higher activity compared to male mice in all groups whereas Zn led to reduced activity. Daily activity rhythm was altered in both the tau and dual Tg mice, and Zn impacted this alteration through effects on amyloid, tau, and through circadian pathways.
Keywords: Alzheimer’s disease; age; circadian activity; mouse model; sex; zinc.
Copyright © 2020 Lippi, Kakalec, Smith and Flinn.
Figures


Similar articles
-
A Novel hAPP/htau Mouse Model of Alzheimer's Disease: Inclusion of APP With Tau Exacerbates Behavioral Deficits and Zinc Administration Heightens Tangle Pathology.Front Aging Neurosci. 2018 Nov 22;10:382. doi: 10.3389/fnagi.2018.00382. eCollection 2018. Front Aging Neurosci. 2018. PMID: 30524268 Free PMC article.
-
The effects of social environment on AD-related pathology in hAPP-J20 mice and tau-P301L mice.Neurobiol Dis. 2023 Oct 15;187:106309. doi: 10.1016/j.nbd.2023.106309. Epub 2023 Sep 23. Neurobiol Dis. 2023. PMID: 37748620
-
Long-term voluntary wheel running does not alter vascular amyloid burden but reduces neuroinflammation in the Tg-SwDI mouse model of cerebral amyloid angiopathy.J Neuroinflammation. 2019 Jul 11;16(1):144. doi: 10.1186/s12974-019-1534-0. J Neuroinflammation. 2019. PMID: 31296239 Free PMC article.
-
Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
-
Modeling Alzheimer's disease in transgenic mice: effect of age and of presenilin1 on amyloid biochemistry and pathology in APP/London mice.Exp Gerontol. 2000 Sep;35(6-7):831-41. doi: 10.1016/s0531-5565(00)00149-2. Exp Gerontol. 2000. PMID: 11053674 Review.
Cited by
-
Role of micronutrients in Alzheimer's disease: Review of available evidence.World J Clin Cases. 2022 Aug 6;10(22):7631-7641. doi: 10.12998/wjcc.v10.i22.7631. World J Clin Cases. 2022. PMID: 36158513 Free PMC article. Review.
References
-
- Alzheimer’s Association (2019). Alzheimer’s Disease Facts and Figures. Chicago, IL: Alzheimer’s Association.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

