Potential Criteria for Frameworks to Support the Evaluation of Innovative Medicines in Upper Middle-Income Countries-A Systematic Literature Review on Value Frameworks and Multi-Criteria Decision Analyses
- PMID: 32922287
- PMCID: PMC7456841
- DOI: 10.3389/fphar.2020.01203
Potential Criteria for Frameworks to Support the Evaluation of Innovative Medicines in Upper Middle-Income Countries-A Systematic Literature Review on Value Frameworks and Multi-Criteria Decision Analyses
Abstract
Background: Multicriteria Decision Analysis (MCDA), a formal decision support framework, has been growing in popularity recently in the field of health care. MCDA can support pricing and reimbursement decisions on the macro level, which is of great importance especially in countries with more limited resources.
Objectives: The aim of this systematic review was to facilitate the development of future MCDA frameworks, by proposing a set of criteria focusing on the purchasing decisions of single-source innovative pharmaceuticals in upper middle-income countries.
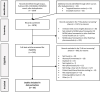
Methods: A systematic literature review was conducted on the decision criteria included in value frameworks (VFs) or MCDA tools. Scopus, Medline, databases of universities, websites of Health Technology Assessment Agencies, and other relevant organizations were included in the search. Double title-abstract screening and double full-text review were conducted, and all extracted data were double-checked. A team of researchers performed the merging and selection process of the extracted criteria.
Results: A total of 1,878 articles entered the title and abstract screening. From these, 341 were eligible to the full-text review, and 36 were included in the final data extraction phase. From these articles 394 criteria were extracted in total. After deduplication and clustering, 26 different criteria were identified. After the merging and selection process, a set of 16 general criteria was proposed.
Conclusion: Based on the results of the systematic literature review, a pool of 16 criteria was selected. This can serve as a starting point for constructing MCDA frameworks in upper middle-income countries after careful adaptation to the local context.
Keywords: developing country; health technology assessment; multiple criteria; pharmaceutical; reimbursement.
Copyright © 2020 Jakab, Németh, Elezbawy, Karadayı, Tozan, Aydın, Shen and Kaló.
Figures
Similar articles
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
Criteria and Scoring Functions Used in Multi-criteria Decision Analysis and Value Frameworks for the Assessment of Rare Disease Therapies: A Systematic Literature Review.Pharmacoecon Open. 2021 Dec;5(4):605-612. doi: 10.1007/s41669-021-00271-w. Epub 2021 May 18. Pharmacoecon Open. 2021. PMID: 34003484 Free PMC article.
-
The application of multi-criteria decision analysis in evaluating the value of drug-oriented intervention: a literature review.Front Pharmacol. 2024 Apr 24;15:1245825. doi: 10.3389/fphar.2024.1245825. eCollection 2024. Front Pharmacol. 2024. PMID: 38720775 Free PMC article. Review.
-
How can multi criteria decision analysis support value assessment of pharmaceuticals? - Findings from a systematic literature review.Expert Rev Pharmacoecon Outcomes Res. 2018 Aug;18(4):379-391. doi: 10.1080/14737167.2018.1467759. Epub 2018 Apr 29. Expert Rev Pharmacoecon Outcomes Res. 2018. PMID: 29707985 Review.
-
Case studies for implementing MCDA for tender and purchasing decisions in hospitals in Indonesia and Thailand.J Pharm Policy Pract. 2021 Jun 14;14(1):52. doi: 10.1186/s40545-021-00333-8. J Pharm Policy Pract. 2021. PMID: 34127071 Free PMC article.
Cited by
-
Patient and Payer Preferences for Additional Value Criteria.Front Pharmacol. 2021 Jun 24;12:690021. doi: 10.3389/fphar.2021.690021. eCollection 2021. Front Pharmacol. 2021. PMID: 34248638 Free PMC article.
-
Early Access to Medicines: Use of Multicriteria Decision Analysis (MCDA) as a Decision Tool in Catalonia (Spain).J Clin Med. 2022 Mar 1;11(5):1353. doi: 10.3390/jcm11051353. J Clin Med. 2022. PMID: 35268443 Free PMC article.
-
How innovation can be defined, evaluated and rewarded in health technology assessment.Health Econ Rev. 2022 Jan 3;12(1):1. doi: 10.1186/s13561-021-00342-y. Health Econ Rev. 2022. PMID: 34981266 Free PMC article. Review.
-
Contextual factors in value-based decision support to enhance health technologies adoption: the case of biosimilars.Front Pharmacol. 2025 Aug 13;16:1599013. doi: 10.3389/fphar.2025.1599013. eCollection 2025. Front Pharmacol. 2025. PMID: 40880644 Free PMC article.
-
Development of the Emirates Multi-Criteria Decision Analysis Tool for Orphan Drugs.Cureus. 2024 Feb 29;16(2):e55215. doi: 10.7759/cureus.55215. eCollection 2024 Feb. Cureus. 2024. PMID: 38558740 Free PMC article.
References
-
- Angelis A., Kanavos P. (2016). Value-based assessment of new medical technologies: towards a robust methodological framework for the application of multiple criteria decision analysis in the context of health technology assessment. Pharmacoeconomics 34 (5), 435–446. 10.1007/s40273-015-0370-z - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources