Isatuximab Acts Through Fc-Dependent, Independent, and Direct Pathways to Kill Multiple Myeloma Cells
- PMID: 32922390
- PMCID: PMC7457083
- DOI: 10.3389/fimmu.2020.01771
Isatuximab Acts Through Fc-Dependent, Independent, and Direct Pathways to Kill Multiple Myeloma Cells
Abstract
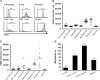
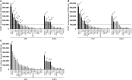
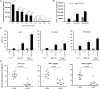
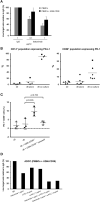
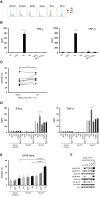
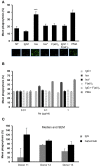
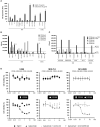
Isatuximab is a monoclonal antibody targeting the transmembrane receptor and ectoenzyme CD38, a protein highly expressed on hematological malignant cells, including those in multiple myeloma (MM). Upon binding to CD38-expressing MM cells, isatuximab is thought to induce tumor cell killing via fragment crystallizable (Fc)-dependent mechanisms, including antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC), as well as via direct Fc-independent mechanisms. Here, these mechanisms of action were investigated in MM and diffuse large B-cell lymphoma (DLBCL) cell lines, as well as in peripheral blood mononuclear cells derived from healthy donors, and in MM patient-derived samples. Our findings show that isatuximab-mediated cytotoxicity occurred primarily via ADCC and ADCP in MM cell lines and via ADCC and apoptosis in DLBCL cell lines expressing high levels of CD38. We identified the programmed cell death-1/programmed cell death-ligand 1 (PD-1/PD-L1) pathway and MM cell-secreted transforming growth factor-beta (TGF-β) as tumor cell-related features that could suppress CD38-mediated ADCC. Furthermore, we established that isatuximab can directly activate natural killer (NK) cells and promote NK cell-mediated cytotoxicity via crosslinking of CD38 and CD16. Finally, isatuximab-induced CDC was observed in cell lines with high CD38 receptor density (>250,000 molecules/cell) and limited expression of inhibitory complement regulatory proteins (CD46, CD55, and CD59; <50,000 molecules/cell). Taken together, our findings highlight mechanistic insights for isatuximab and provide support for a range of combination therapy approaches that could be tested for isatuximab in the future.
Keywords: CD38; PD-1; TGF-β; antibody-dependent cellular cytotoxicity; antibody-dependent cellular phagocytosis; isatuximab; multiple myeloma; natural killer cells.
Copyright © 2020 Zhu, Song, Wang, Srinivasan, Yang, Greco, Theilhaber, Shehu, Wu, Yang, Passe-Coutrin, Fournier, Tai, Anderson, Wiederschain, Bahjat, Adrián and Chiron.
Figures
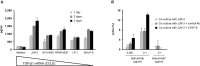
References
-
- Deaglio S, Morra M, Mallone R, Ausiello CM, Prager E, Garbarino G, et al. Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an Ig superfamily member. J Immunol. (1998) 160:395–402. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

