CR4 Signaling Contributes to a DC-Driven Enhanced Immune Response Against Complement-Opsonized HIV-1
- PMID: 32922405
- PMCID: PMC7457048
- DOI: 10.3389/fimmu.2020.02010
CR4 Signaling Contributes to a DC-Driven Enhanced Immune Response Against Complement-Opsonized HIV-1
Abstract
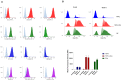
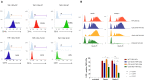
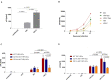
Dendritic cells (DCs) possess intrinsic cellular defense mechanisms to specifically inhibit HIV-1 replication. In turn, HIV-1 has evolved strategies to evade innate immune sensing by DCs resulting in suboptimal maturation and poor antiviral immune responses. We previously showed that complement-opsonized HIV-1 (HIV-C) was able to efficiently infect various DC subsets significantly higher than non-opsonized HIV-1 (HIV) and therefore also mediate a higher antiviral immunity. Thus, complement coating of HIV-1 might play a role with respect to viral control occurring early during infection via modulation of DCs. To determine in detail which complement receptors (CRs) expressed on DCs was responsible for infection and superior pro-inflammatory and antiviral effects, we generated stable deletion mutants for the α-chains of CR3, CD11b, and CR4, CD11c using CRISPR/Cas9 in THP1-derived DCs. We found that CD11c deletion resulted in impaired DC infection as well as antiviral and pro-inflammatory immunity upon exposure to complement-coated HIV-1. In contrast, sole expression of CD11b on DCs shifted the cells to an anti-inflammatory, regulatory DC type. We here illustrated that CR4 comprised of CD11c and CD18 is the major player with respect to DC infection associated with a potent early pro-inflammatory immune response. A more detailed characterization of CR3 and CR4 functions using our powerful tool might open novel avenues for early therapeutic intervention during HIV-1 infection.
Keywords: CD11b; CD11c; HIV-1; complement; dendritic cell.
Copyright © 2020 Bermejo-Jambrina, Blatzer, Jauregui-Onieva, Yordanov, Hörtnagl, Valovka, Huber, Wilflingseder and Posch.
Figures


Similar articles
-
Role of Complement Receptors (CRs) on DCs in Anti-HIV-1 Immunity.Front Immunol. 2020 Nov 3;11:572114. doi: 10.3389/fimmu.2020.572114. eCollection 2020. Front Immunol. 2020. PMID: 33224139 Free PMC article. Review.
-
Complement Potentiates Immune Sensing of HIV-1 and Early Type I Interferon Responses.mBio. 2021 Oct 26;12(5):e0240821. doi: 10.1128/mBio.02408-21. Epub 2021 Oct 12. mBio. 2021. PMID: 34634939 Free PMC article.
-
The role of CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in complement-mediated phagocytosis and podosome formation by human phagocytes.Immunol Lett. 2017 Sep;189:64-72. doi: 10.1016/j.imlet.2017.05.014. Epub 2017 May 26. Immunol Lett. 2017. PMID: 28554712
-
Dysfunction of complement receptors CR3 (CD11b/18) and CR4 (CD11c/18) in pre-eclampsia: a genetic and functional study.BJOG. 2021 Jul;128(8):1282-1291. doi: 10.1111/1471-0528.16660. Epub 2021 Mar 14. BJOG. 2021. PMID: 33539617 Free PMC article.
-
Biologia Futura: stories about the functions of β2-integrins in human phagocytes.Biol Futur. 2021 Mar;72(1):7-13. doi: 10.1007/s42977-020-00063-z. Epub 2021 Feb 6. Biol Futur. 2021. PMID: 34554501 Review.
Cited by
-
Control of complement-induced inflammatory responses to SARS-CoV-2 infection by anti-SARS-CoV-2 antibodies.EMBO J. 2024 Apr;43(7):1135-1163. doi: 10.1038/s44318-024-00061-0. Epub 2024 Feb 28. EMBO J. 2024. PMID: 38418557 Free PMC article.
-
HIV-1 Trans Infection via TNTs Is Impeded by Targeting C5aR.Biomolecules. 2022 Feb 15;12(2):313. doi: 10.3390/biom12020313. Biomolecules. 2022. PMID: 35204813 Free PMC article.
-
Role of Complement Receptors (CRs) on DCs in Anti-HIV-1 Immunity.Front Immunol. 2020 Nov 3;11:572114. doi: 10.3389/fimmu.2020.572114. eCollection 2020. Front Immunol. 2020. PMID: 33224139 Free PMC article. Review.
-
The complement system in human pregnancy and preeclampsia.Front Immunol. 2025 Aug 19;16:1617140. doi: 10.3389/fimmu.2025.1617140. eCollection 2025. Front Immunol. 2025. PMID: 40904461 Free PMC article. Review.
-
Complement-Opsonized HIV Modulates Pathways Involved in Infection of Cervical Mucosal Tissues: A Transcriptomic and Proteomic Study.Front Immunol. 2021 May 20;12:625649. doi: 10.3389/fimmu.2021.625649. eCollection 2021. Front Immunol. 2021. PMID: 34093520 Free PMC article.
References
-
- Ebenbichler CF, Thielens NM, Vornhagen R, Marschang P, Arlaud GJ, Dierich MP. Human immunodeficiency virus type 1 activates the classical pathway of complement by direct C1 binding through specific sites in the transmembrane glycoprotein gp41. J Exp Med. (1991) 174:1417–24. 10.1084/jem.174.6.1417 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

