Acyltransferases Regulate Oil Quality in Camelina sativa Through Both Acyl Donor and Acyl Acceptor Specificities
- PMID: 32922411
- PMCID: PMC7456936
- DOI: 10.3389/fpls.2020.01144
Acyltransferases Regulate Oil Quality in Camelina sativa Through Both Acyl Donor and Acyl Acceptor Specificities
Abstract
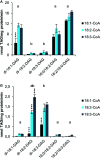
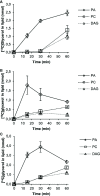
Camelina sativa is an emerging biotechnology oil crop. However, more information is needed regarding its innate lipid enzyme specificities. We have therefore characterized several triacylglycerol (TAG) producing enzymes by measuring in vitro substrate specificities using different combinations of acyl-acceptors (diacylglycerol, DAG) and donors. Specifically, C. sativa acyl-CoA:diacylglycerol acyltransferase (DGAT) 1 and 2 (which both use acyl-CoA as acyl donor) and phospholipid:diacylglycerol acyltransferase (PDAT, with phosphatidylcoline as acyl donor) were studied. The results show that the DGAT1 and DGAT2 specificities are complementary, with DGAT2 exhibiting a high specificity for acyl acceptors containing only polyunsaturated fatty acids (FAs), whereas DGAT1 prefers acyl donors with saturated and monounsaturated FAs. Furthermore, the combination of substrates that resulted in the highest activity for DGAT2, but very low activity for DGAT1, corresponds to TAG species previously shown to increase in C. sativa seeds with downregulated DGAT1. Similarly, the combinations of substrates that gave the highest PDAT1 activity were also those that produce the two TAG species (54:7 and 54:8 TAG) with the highest increase in PDAT overexpressing C. sativa seeds. Thus, the in vitro data correlate well with the changes in the overall fatty acid profile and TAG species in C. sativa seeds with altered DGAT1 and PDAT activity. Additionally, in vitro studies of C. sativa phosphatidycholine:diacylglycerol cholinephosphotransferase (PDCT), another activity involved in TAG biosynthesis, revealed that PDCT accepts substrates with different desaturation levels. Furthermore, PDCT was unable to use DAG with ricineoleyl groups, and the presence of this substrate also inhibited PDCT from using other DAG-moieties. This gives insights relating to previous in vivo studies regarding this enzyme.
Keywords: Camelina; Kennedy pathway; acyltransferase; diacylglycerol; diacylglycerol acyltransferase; fatty acid composition; phosphatidylcholine; triacylglycerol.
Copyright © 2020 Lager, Jeppson, Gippert, Feussner, Stymne and Marmon.
Figures




Similar articles
-
Camelina sativa phosphatidylcholine:diacylglycerol cholinephosphotransferase-catalyzed interconversion does not discriminate between substrates.Lipids. 2021 Nov;56(6):591-602. doi: 10.1002/lipd.12322. Epub 2021 Aug 31. Lipids. 2021. PMID: 34463366
-
Activities of acyl-CoA:diacylglycerol acyltransferase (DGAT) and phospholipid:diacylglycerol acyltransferase (PDAT) in microsomal preparations of developing sunflower and safflower seeds.Planta. 2013 Jun;237(6):1627-36. doi: 10.1007/s00425-013-1870-8. Epub 2013 Mar 29. Planta. 2013. PMID: 23539042 Free PMC article.
-
Two Acyltransferases Contribute Differently to Linolenic Acid Levels in Seed Oil.Plant Physiol. 2017 Apr;173(4):2081-2095. doi: 10.1104/pp.16.01865. Epub 2017 Feb 24. Plant Physiol. 2017. PMID: 28235891 Free PMC article.
-
Properties and Biotechnological Applications of Acyl-CoA:diacylglycerol Acyltransferase and Phospholipid:diacylglycerol Acyltransferase from Terrestrial Plants and Microalgae.Lipids. 2018 Jul;53(7):663-688. doi: 10.1002/lipd.12081. Epub 2018 Sep 25. Lipids. 2018. PMID: 30252128 Review.
-
Advancing oleaginous microorganisms to produce lipid via metabolic engineering technology.Prog Lipid Res. 2013 Oct;52(4):395-408. doi: 10.1016/j.plipres.2013.05.002. Epub 2013 May 16. Prog Lipid Res. 2013. PMID: 23685199 Review.
Cited by
-
Phosphatidylcholine:diacylglycerol cholinephosphotransferase's unique regulation of castor bean oil quality.Plant Physiol. 2022 Aug 1;189(4):2001-2014. doi: 10.1093/plphys/kiac209. Plant Physiol. 2022. PMID: 35522031 Free PMC article.
-
LPEATs Tailor Plant Phospholipid Composition through Adjusting Substrate Preferences to Temperature.Int J Mol Sci. 2021 Jul 29;22(15):8137. doi: 10.3390/ijms22158137. Int J Mol Sci. 2021. PMID: 34360902 Free PMC article.
-
Kinetic complexities of triacylglycerol accumulation in developing embryos from Camelina sativa provide evidence for multiple biosynthetic systems.J Biol Chem. 2022 Jan;298(1):101396. doi: 10.1016/j.jbc.2021.101396. Epub 2021 Nov 12. J Biol Chem. 2022. PMID: 34774796 Free PMC article.
-
Towards rational control of seed oil composition: dissecting cellular organization and flux control of lipid metabolism.Plant Physiol. 2025 Feb 7;197(2):kiae658. doi: 10.1093/plphys/kiae658. Plant Physiol. 2025. PMID: 39657632 Free PMC article. Review.
-
Suppression of Physaria fendleri SDP1 Increased Seed Oil and Hydroxy Fatty Acid Content While Maintaining Oil Biosynthesis Through Triacylglycerol Remodeling.Front Plant Sci. 2022 Jun 3;13:931310. doi: 10.3389/fpls.2022.931310. eCollection 2022. Front Plant Sci. 2022. PMID: 35720575 Free PMC article.
References
-
- Banaś W., Garcia A. S., Banaś A., Stymne S. (2013). Activities of acyl-CoA: diacylglycerol acyltransferase (DGAT) and phospholipid: diacylglycerol acyltransferase (PDAT) in microsomal preparations of developing sunflower and safflower seeds. Planta 237 (6), 1627–1636. 10.1007/s00425-013-1870-8 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

