The effect of electronic monitoring combined with weekly feedback and reminders on adherence to inhaled corticosteroids in infants and younger children with asthma: a randomized controlled trial
- PMID: 32922454
- PMCID: PMC7477854
- DOI: 10.1186/s13223-020-00466-6
The effect of electronic monitoring combined with weekly feedback and reminders on adherence to inhaled corticosteroids in infants and younger children with asthma: a randomized controlled trial
Abstract
Background: Adherence to asthma treatment among children is usually poor. We sought to explore whether electronic adherence monitoring combined with weekly feedback regarding adherence along with a reminder to use inhaled corticosteroids (ICS) would lead to improved compliance with ICS in infants and younger children with asthma.
Methods: 96 recruited children (aged 6 months to 3 years) with mild or moderate persistent asthma who were on regular inhaled corticosteroids were randomly allocated to receive electronic monitoring combined with instant messaging software (IMS)-based weekly feedback regarding adherence along with a reminder to keep taking the ICS (intervention group) and to receive electronic monitoring only (control group).
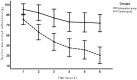
Results: The mean device-monitored adherence was significantly higher in the intervention group (80%) than in the control group (45.9%), with a difference of 34.0% (95% confidence interval [CI], 26.8-41.3%; P < 0.001). No difference in the mean caregiver-reported adherence between the interventional group (89.7%) and the control group (92.7%) was observed (P = 0.452).
Conclusions: Electronic monitoring combined with IMS-based weekly feedback regarding adherence along with a reminder to keep taking the ICS significantly improved the treatment compliance of infants and younger children with asthma. Caregiver-reported adherence is an unreliable monitoring indicator.Trial registration ClinicalTrials.gov, NCT03277664. Registered 11 September 2017-Retrospectively registered, https://clinicaltrials.gov/ct2/results?cond=&term=NCT03277664.
Keywords: Adherence; Asthma; Children; Electronic device.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures


References
-
- Williams LK, Peterson EL, Wells K, Ahmedani BK, Kumar R, Burchard EG, Chowdhry VK, Favro D, Lanfear DE, Pladevall M. Quantifying the proportion of severe asthma exacerbations attributable to inhaled corticosteroid nonadherence. J Allergy Clin Immunol. 2011;128(6):1185–1191.e1182. doi: 10.1016/j.jaci.2011.09.011. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

