Outcomes of patients with relapsed or refractory acute myeloid leukemia: a population-based real-world study
- PMID: 32923092
- PMCID: PMC7486485
Outcomes of patients with relapsed or refractory acute myeloid leukemia: a population-based real-world study
Abstract
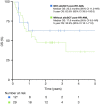
With standard therapies for patients with acute myeloid leukemia (AML), many patients either do not achieve complete response (CR) or relapse after CR. There are a scarcity of real-world data on outcomes of unselected patients with relapsed/refractory acute myeloid leukemia (RR-AML). We retrospectively evaluated treatment patterns and survival outcomes of unselected patients aged ≥18 years diagnosed with RR-AML identified from the Alberta Cancer Registry, Alberta, Canada, between January 2013 and December 2016. We included 199 patients who met predefined criteria for RR-AML. Following RR-AML diagnosis, 23% of patients received intensive therapy (IT), 33% non-intensive therapy (NIT), and 44% best supportive care (BSC). The unadjusted median overall survival (OS) of the study cohort was 5.3 months from the time of RR-AML diagnosis, with a 5-year OS rate of 12.6% (95% confidence interval 7.5-21.1). According to treatment intensity after RR-AML, the median OS outcomes were 13.6, 9.4, and 2.0 months for IT, NIT, and BSC groups, respectively (P<0.001). Patients who received treatment (IT or NIT) had better survival than those who received only BSC. This study emphasizes the need for newer therapy options for patients with RR-AML.
Keywords: Acute myeloid leukemia; overall survival; real-world data; refractory; registry; relapsed.
AJBR Copyright © 2020.
Conflict of interest statement
JMB has received honoraria from Bristol Myers Squibb, Novartis, Otsuka, Pfizer, Jazz Pharmaceuticals and Teva. MNG has received honoraria from Bristol Myers Squibb, Novartis, Otsuka, Pfizer, Jazz Pharmaceuticals. DY has received honoraria from Bristol Myers Squibb to conduct this study. FFL is an employee of Bristol Myers Squibb. CW is an employee of Celgene Inc., a Bristol Myers Squibb company. WYC has received research funding and consulting fees from Bristol Myers Squibb. The remaining authors have no conflicts of interest to disclose.
Figures


References
-
- O’Donnell MR, Abboud CN, Altman J, Appelbaum FR, Arber DA, Attar E, Borate U, Coutre SE, Damon LE, Goorha S, Lancet J, Maness LJ, Marcucci G, Millenson MM, Moore JO, Ravandi F, Shami PJ, Smith BD, Stone RM, Strickland SA, Tallman MS, Wang ES, Naganuma M, Gregory KM. Acute myeloid leukemia. J Natl Compr Canc Netw. 2012;10:984–1021. - PubMed
-
- Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, Wheatley K, Harrison CJ, Burnett AK. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities amongst 5876 younger adult patients treated in the UK Medical Research Council trials. Blood. 2010;116:354–365. - PubMed
-
- Burnett AK, Milligan D, Prentice AG, Goldstone AH, McMullin MF, Hills RK, Wheatley K. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109:1114–1124. - PubMed
-
- Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, Kumar R, Cavenagh J, Schuh AC, Candoni A, Récher C, Sandhu I, Bernal del Castillo T, Al-Ali HK, Martinelli G, Falantes J, Noppeney R, Stone RM, Minden MD, McIntyre H, Songer S, Lucy LM, Beach CL, Döhner H. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126:291–299. - PMC - PubMed
-
- Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, Gau JP, Chou WC, Buckstein R, Cermak J, Kuo CY, Oriol A, Ravandi F, Faderl S, Delaunay J, Lysák D, Minden M, Arthur C. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J. Clin. Oncol. 2012;30:2670–2677. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
