Identification of a neo-epitope dominating endogenous CD8 T cell responses to MC-38 colorectal cancer
- PMID: 32923109
- PMCID: PMC7458608
- DOI: 10.1080/2162402X.2019.1673125
Identification of a neo-epitope dominating endogenous CD8 T cell responses to MC-38 colorectal cancer
Abstract
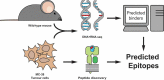
The murine MC-38 colorectal cancer model is a commonly used model for cancer with high mutational burden, which is sensitive for immune checkpoint immunotherapy. We set out to analyze endogenous CD8+ T cell responses to MC-38 neo-antigens in tumor-bearing mice and after anti-PD-L1 checkpoint therapy. Through combination of whole-exome sequencing analysis with mass spectrometry of MHC class I eluted peptides we could identify eight candidate epitopes. Of these, a neo-epitope encoded by a point-mutation in the sequence of the ribosomal protein L18 (Rpl18) strongly dominated the CD8+ T cell response to our MC-38 cell-line in comparison to a previously described neo-epitope in the Adpgk protein. Therapeutic vaccination with synthetic peptides induced CD8+ T cell responses against the mutated Rpl18 epitope, which controlled tumor growth in vivo. This immunologically dominant response to mutated Rpl18 is of great importance in the development and optimization of immunotherapeutic strategies with the MC-38 tumor model.
Keywords: Neoantigen; PD-L1; checkpoint; immunotherapy; vaccination; whole exome sequencing.
© 2020 The Author(s). Published with license by Taylor & Francis Group, LLC.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
