The metabolic enzyme arginase-2 is a potential target for novel immune modulatory vaccines
- PMID: 32923127
- PMCID: PMC7458644
- DOI: 10.1080/2162402X.2020.1771142
The metabolic enzyme arginase-2 is a potential target for novel immune modulatory vaccines
Abstract
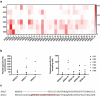
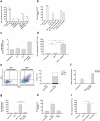
One way that tumors evade immune destruction is through tumor and stromal cell expression of arginine-degrading enzyme arginase-2 (ARG2). Here we describe the existence of pro-inflammatory effector T-cells that recognize ARG2 and can directly target tumor and tumor-infiltrating cells. Using a library of 34 peptides covering the entire ARG2 sequence, we examined reactivity toward these peptides in peripheral blood mononuclear cells from cancer patients and healthy individuals. Interferon-γ ELISPOT revealed frequent immune responses against several of the peptides, indicating that ARG2-specific self-reactive T-cells are natural components of the human T-cell repertoire. Based on this, the most immunogenic ARG2 protein region was further characterized. By identifying conditions in the microenvironment that induce ARG2 expression in myeloid cells, we showed that ARG2-specific CD4T-cells isolated and expanded from a peripheral pool from a prostate cancer patient could recognize target cells in an ARG2-dependent manner. In the 'cold' in vivo tumor model Lewis lung carcinoma, we found that activation of ARG2-specific T-cells by vaccination significantly inhibited tumor growth. Immune-modulatory vaccines targeting ARG2 thus are a candidate strategy for cancer immunotherapy.
Keywords: ARG2; Anti-Tregs; T-win; immune modulation.
© 2020 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
MHA has made an invention based on the use of ARG2 for vaccinations. The rights of the invention have been transferred to Copenhagen University Hospital Herlev, according to the Danish Law of Public Inventions at Public Research Institutions. The capital region has licensed the rights to the company IO Biotech ApS, whose purpose is to develop immune-modulating vaccines for cancer treatments. The patent application was filed by IO Biotech ApS. MHA is a shareholder and board member of IO Biotech ApS. IMS is a shareholder of IO Biotech ApS. EM and AWP are employed at IO Biotech ApS.
Figures






References
-
- Mondanelli G, Bianchi R, Pallotta MT, Orabona C, Albini E, Iacono A, Belladonna ML, Vacca C, Fallarino F, Macchiarulo A, et al. A relay pathway between arginine and tryptophan metabolism confers immunosuppressive properties on dendritic cells. Immunity. 2017;46(2):233–244. doi:10.1016/j.immuni.2017.01.005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
