A liposomal RNA vaccine inducing neoantigen-specific CD4+ T cells augments the antitumor activity of local radiotherapy in mice
- PMID: 32923128
- PMCID: PMC7458669
- DOI: 10.1080/2162402X.2020.1771925
A liposomal RNA vaccine inducing neoantigen-specific CD4+ T cells augments the antitumor activity of local radiotherapy in mice
Abstract
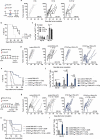
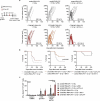
Antigen-encoding, lipoplex-formulated RNA (RNA-LPX) enables systemic delivery to lymphoid compartments and selective expression in resident antigen-presenting cells. We report here that the rejection of CT26 tumors, mediated by local radiotherapy (LRT), is further augmented in a CD8+ T cell-dependent manner by an RNA-LPX vaccine that encodes CD4+ T cell-recognized neoantigens (CD4 neoantigen vaccine). Whereas CD8+ T cells induced by LRT alone were primarily directed against the immunodominant gp70 antigen, mice treated with LRT plus the CD4 neoantigen vaccine rejected gp70-negative tumors and were protected from rechallenge with these tumors, indicating a potent poly-antigenic CD8+ T cell response and T cell memory. In the spleens of CD4 neoantigen-vaccinated mice, we found a high number of activated, poly-functional, Th1-like CD4+ T cells against ME1, the immunodominant CD4 neoantigen within the poly-neoantigen vaccine. LRT itself strongly increased CD8+ T cell numbers and clonal expansion. However, tumor infiltrates of mice treated with CD4 neoantigen vaccine/LRT, as compared to LRT alone, displayed a higher fraction of activated gp70-specific CD8+ T cells, lower PD-1/LAG-3 expression and contained ME1-specific IFNγ+ CD4+ T cells capable of providing cognate help. CD4 neoantigen vaccine/LRT treatment followed by anti-CTLA-4 antibody therapy further enhanced the efficacy with complete remission of gp70-negative CT26 tumors and survival of all mice. Our data highlight the power of combining synergistic modes of action and warrants further exploration of the presented treatment schema.
Keywords: CD4+ T cells; RNA-LPX; Radiotherapy; cancer vaccines; neoantigens.
© 2020 The Author(s). Published with license by Taylor & Francis Group, LLC.
Figures



References
-
- Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, Schwendener R, Pruschy M, Knuth A, van den Broek M, et al. Radiotherapy promotes tumor-specific effector cd8+t cells via dendritic cell activation. The Journal of Immunology. 2012;189(2):558–13. (Baltimore, Md.: 1950). doi:10.4049/jimmunol.1200563. - DOI - PubMed
-
- Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li X-D, Mauceri H, Beckett M, Darga T, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type i interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41(5):843–852. doi:10.1016/j.immuni.2014.10.019. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
