Adoptive cell therapy of triple negative breast cancer with redirected cytokine-induced killer cells
- PMID: 32923140
- PMCID: PMC7458615
- DOI: 10.1080/2162402X.2020.1777046
Adoptive cell therapy of triple negative breast cancer with redirected cytokine-induced killer cells
Abstract
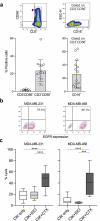
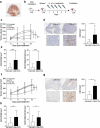
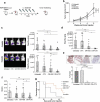
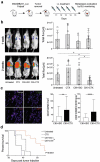
Cytokine-Induced Killer (CIK) cells share several functional and phenotypical properties of both T and natural killer (NK) cells. They represent an attractive approach for cell-based immunotherapy, as they do not require antigen-specific priming for tumor cell recognition, and can be rapidly expanded in vitro. Their relevant expression of FcγRIIIa (CD16a) can be exploited in combination with clinical-grade monoclonal antibodies (mAbs) to redirect their lytic activity in an antigen-specific manner. Here, we report the efficacy of this combined approach against triple negative breast cancer (TNBC), an aggressive tumor that still requires therapeutic options. Different primitive and metastatic TNBC cancer mouse models were established in NSG mice, either by implanting patient-derived TNBC samples or injecting MDA-MB-231 cells orthotopically or intravenously. The combined treatment consisted in the repeated intratumoral or intravenous injection of CIK cells and cetuximab. Tumor growth and metastasis were monitored by bioluminescence or immunohistochemistry, and survival was recorded. CIK cells plus cetuximab significantly restrained primitive tumor growth in mice, either in patient-derived tumor xenografts or MDA-MB-231 cell line models. Moreover, this approach almost completely abolished metastasis spreading and dramatically improved survival. The antigen-specific mAb favored tumor and metastasis tissue infiltration by CIK cells, and led to an enrichment of the CD16a+ subset.Data highlight the potentiality of this novel immunotherapy strategy where a nonspecific cytotoxic cell population can be converted into tumor-specific effectors with clinical-grade antibodies, thus providing not only a therapeutic option for TNBC but also a valid alternative to more complex approaches based on chimeric antigen receptor-engineered cells.
List of abbreviations: ACT, Adoptive Cell Transfer; ADCC, Antibody-Dependent Cell-mediated Cytotoxicity; ADP, Adenosine diphosphate; BLI, Bioluminescence Imaging; CAR, Chimeric Antigen Receptor; CIK, Cytokine Induced Killer cells; CTX, Cetuximab; DMEM, Dulbecco's Modified Eagle Medium; EGFR, Human Epidermal Growth Factor 1; ER, Estrogen; FBS, Fetal Bovine Serum; FFPE, Formalin-Fixed Paraffin-Embedded; GMP, Good Manufacturing Practices; GVHD, Graft Versus Host Disease; HER2, Human Epidermal Growth Factor 2; HRP, Horseradish Peroxidase; IFN-γ, Interferon-γ; IHC, Immunohistochemistry; IL-2, Interleukin-2; ISO, Irrelevant antibody; i.t., intratumoral; i.v., intravenous, mAbs, Monoclonal Antibodies; mIHC, Multiplex Fluorescence Immunohistochemistry; MHC, Major Histocompatibility Complex; NK, Natural Killer; NKG2D, Natural-Killer group 2 member D; NSG, NOD/SCID common γ chain knockout; PARP, Poly ADP-ribose polymerase; PBMCs, Peripheral Blood Mononuclear Cells; PBS, Phosphate-buffered saline; PDX, Patient-derived xenograft; PR, Progesterone; rhIFN-γ, Recombinant Human Interferon-γ; RPMI, Roswell Park Memorial Institute; STR, Short tandem Repeat; TCR, T Cell Receptor; TNBC, Triple Negative Breast Cancer; TSA, Tyramide Signal Amplification.
Keywords: Cytokine-induced killer cells (CIK); TNBC mouse models; adoptive cells therapy (ACT); cetuximab; triple negative breast cancer (TNBC).
© 2020 The Author(s). Published with license by Taylor & Francis Group, LLC.
Figures

Similar articles
-
Chimeric antigen receptor-engineered cytokine-induced killer cells overcome treatment resistance of pre-B-cell acute lymphoblastic leukemia and enhance survival.Int J Cancer. 2016 Oct 15;139(8):1799-809. doi: 10.1002/ijc.30217. Epub 2016 Jun 11. Int J Cancer. 2016. PMID: 27253354
-
ERBB2-CAR-Engineered Cytokine-Induced Killer Cells Exhibit Both CAR-Mediated and Innate Immunity Against High-Risk Rhabdomyosarcoma.Front Immunol. 2020 Oct 19;11:581468. doi: 10.3389/fimmu.2020.581468. eCollection 2020. Front Immunol. 2020. PMID: 33193388 Free PMC article.
-
Targeting epidermal growth factor-overexpressing triple-negative breast cancer by natural killer cells expressing a specific chimeric antigen receptor.Cell Prolif. 2020 Aug;53(8):e12858. doi: 10.1111/cpr.12858. Epub 2020 Jun 27. Cell Prolif. 2020. PMID: 32592435 Free PMC article.
-
How can Cytokine-induced killer cells overcome CAR-T cell limits.Front Immunol. 2023 Aug 22;14:1229540. doi: 10.3389/fimmu.2023.1229540. eCollection 2023. Front Immunol. 2023. PMID: 37675107 Free PMC article. Review.
-
The dual-functional capability of cytokine-induced killer cells and application in tumor immunology.Hum Immunol. 2015 May;76(5):385-91. doi: 10.1016/j.humimm.2014.09.021. Epub 2014 Oct 8. Hum Immunol. 2015. PMID: 25305457 Review.
Cited by
-
Immunotherapy and immunoengineering for breast cancer; a comprehensive insight into CAR-T cell therapy advancements, challenges and prospects.Cell Oncol (Dordr). 2022 Oct;45(5):755-777. doi: 10.1007/s13402-022-00700-w. Epub 2022 Aug 9. Cell Oncol (Dordr). 2022. PMID: 35943716 Review.
-
Immunotherapy in hematologic malignancies: achievements, challenges and future prospects.Signal Transduct Target Ther. 2023 Aug 18;8(1):306. doi: 10.1038/s41392-023-01521-5. Signal Transduct Target Ther. 2023. PMID: 37591844 Free PMC article. Review.
-
Cytokine-induced killer (CIK) cells, successes and challenges: report on the first international conference dedicated to the clinical translation of this unique adoptive cell immunotherapy.Cancer Immunol Immunother. 2024 Jan 27;73(2):21. doi: 10.1007/s00262-023-03605-1. Cancer Immunol Immunother. 2024. PMID: 38279995 Free PMC article.
-
Cannabinoids and triple-negative breast cancer treatment.Front Immunol. 2024 Aug 8;15:1386548. doi: 10.3389/fimmu.2024.1386548. eCollection 2024. Front Immunol. 2024. PMID: 39176080 Free PMC article. Review.
-
Intratumoral delivery of immunotherapy to treat breast cancer: current development in clinical and preclinical studies.Front Immunol. 2024 May 13;15:1385484. doi: 10.3389/fimmu.2024.1385484. eCollection 2024. Front Immunol. 2024. PMID: 38803496 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
