Recombinant viruses delivering the necroptosis mediator MLKL induce a potent antitumor immunity in mice
- PMID: 32923163
- PMCID: PMC7458643
- DOI: 10.1080/2162402X.2020.1802968
Recombinant viruses delivering the necroptosis mediator MLKL induce a potent antitumor immunity in mice
Abstract
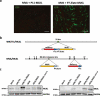
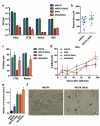
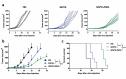
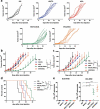
Vaccinia viruses (VACV) are a novel class of immune-oncolytic therapeutics and their mechanism of action is based both on their capacity to replicate selectively in cancer cells and to elicit danger signals that can boost anti-tumor immunity. We recently reported that the intratumor expression of MLKL, a necroptosis inducing factor, generates a protective anti-tumor immunity. Here, we combined both approaches to test the use of VACV to deliver MLKL into the tumor. We generated VACV vectors expressing MLKL and evaluated the effects of MLKL on antitumor efficacy. In vitro infection of cancer cells with MLKL-expressing vectors led to cell death with necroptotic hallmarks. In syngeneic mouse tumor models, VACV expressing MLKL induced an outstanding antitumor activity, which was associated with a robust immunity directed against neo-epitopes. In conclusion, delivery of MLKL by VACV vectors boosts the intrinsic anti-tumor properties of these viral vectors by promoting in situ immunogenic cell death of infected cancer cells.
Keywords: Immunotherapy; MLKL; VACV; necroptosis; oncolytic virus.
© 2020 The Author(s). Published with license by Taylor & Francis Group, LLC.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
